DP Chemistry Questionbank

Options
| Path: |
Description
[N/A]Directly related questions
-
16N.3.sl.TZ0.4e:
Deduce the charge on the indium ion and the formula of indium sulfate.
- 16N.3.sl.TZ0.7b: Explain the effect of increasing the temperature of a nematic liquid crystal on its directional...
-
16N.3.sl.TZ0.20b:
Methadone is sometimes used to help reduce withdrawal symptoms in the treatment of heroin addiction. Outline one withdrawal symptom that an addict may experience.
- 16N.3.hl.TZ0.26c: Omeprazole exists as a racemic mixture whereas esomeprazole is a single enantiomer. Outline how,...
- 16N.3.sl.TZ0.8a: Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed...
-
16N.3.hl.TZ0.21a:
The Geobacter species of bacteria can be used in microbial fuel cells to oxidise aqueous ethanoate ions,
CH3COO−(aq), to carbon dioxide gas.State the half-equations for the reactions at both electrodes.
-
16N.3.hl.TZ0.21c:
Dye-sensitized solar cells (DSSC) convert solar energy into electrical energy.
(i) Describe how a DSSC converts sunlight into electrical energy.
(ii) Explain the role of the electrolyte solution containing iodide ions, I−, and triiodide ions, I3−, in the DSSC.
-
16N.3.hl.TZ0.28a:
Deduce equations for the following nuclear reactions:
(i) Molybdenum-98 absorbs a neutron.
(ii) The isotope produced in (a) (i) decays into technetium-99m.
-
16N.3.hl.TZ0.10b:
Adsorption and chelation are two methods of removing heavy metal ion pollution from the environment.
(i) Describe the process of adsorption.
(ii) Deduce the structure of the complex ion formed by the reaction of three H2N−CH2−CH2−NH2 chelating molecules with a mercury(II) ion.
- 16N.3.hl.TZ0.28b: Molybdenum-99 has a half-life of 66 hours, while technetium-99m has a half-life of 6 hours....
-
16N.3.sl.TZ0.11a:
(i) Calculate the specific energy of the lithium ion battery, in MJ kg−1, when 80.0 kg of fuel in the battery releases 1.58 × 107 J. Use section 1 of the data booklet.
(ii) The specific energy of gasoline is 46.0 MJ kg−1. Suggest why gasoline may be considered a better energy source than the lithium ion battery based on your answer to part (a) (i).
-
16N.3.sl.TZ0.13a:
Explain the effect of the increasing concentration of atmospheric carbon dioxide on the acidity of oceans.
-
16N.3.sl.TZ0.9b:
The structures of two molecules, X and Y, are shown below.
(i) Justify why both these molecules are carbohydrates.
(ii) Distinguish between these molecules in terms of their functional groups.
- 16N.3.sl.TZ0.14a: State the equation for the complete transesterification of the triglyceride given below with...
-
16N.3.sl.TZ0.19c:
A different approach to treating excess stomach acid is to neutralize it with antacids. Formulate an equation that shows the action of an antacid that can neutralize three moles of hydrogen ions, H+, per mole of antacid.
-
16N.3.hl.TZ0.8a:
(i) The diagram below shows the diffraction of two X-ray beams, y and z of wavelength λ, shining on a chromium crystal whose planes are a distance d nm apart.
Deduce the extra distance travelled by the second beam, z, compared to the first one, y.
(ii) State the Bragg’s condition for the observed diffraction to be at its strongest (constructive interference).
- 16N.3.hl.TZ0.9b: Outline one difference between type 1 and type 2 superconductors.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
-
16N.3.sl.TZ0.10b:
A mixture of amino acids is separated by gel electrophoresis at pH 6.0. The amino acids are then stained with ninhydrin.
(i) On the diagram below draw the relative positions of the following amino acids at the end of the process: Val, Asp, Lys and Thr.
(ii) Suggest why glycine and isoleucine separate slightly at pH 6.5.
- 16N.3.hl.TZ0.10a: Compare and contrast the Fenton and Haber–Weiss reaction mechanisms.
-
16N.3.hl.TZ0.13c:
Amino acids act as buffers in solution. In aspartic acid, the side chain (R group) carboxyl has pKa = 4.0. Determine the percentage of the side chain carboxyl that will be ionized (–COO–) in a solution of aspartic acid with pH = 3.0. Use section 1 of the data booklet.
- 16N.3.hl.TZ0.28d: Outline the nature of the radioactive waste that is generated by the use of technetium-99m in...
-
16N.3.sl.TZ0.6a:
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
-
16N.3.sl.TZ0.10d:
The fibrous protein keratin has a secondary structure with a helical arrangement.
(i) State the type of interaction responsible for holding the protein in this arrangement.
(ii) Identify the functional groups responsible for these interactions.
-
16N.3.sl.TZ0.13b:
(i) Describe the changes that occur at the molecular level when atmospheric carbon dioxide gas absorbs infrared radiation emitted from the Earth’s surface.
(ii) Other than changes to the acidity of oceans, suggest why the production of carbon dioxide is of greater concern than the production of water vapour.
-
16N.3.sl.TZ0.15b:
Radioactive phosphorus, 33P, has a half-life of 25.3 days.
(i) Calculate 33P decay constant λ and state its unit. Use section 1 of the data booklet.
(ii) Determine the fraction of the 33P sample remaining after 101.2 days.
-
16N.3.sl.TZ0.9c:
Amylose is an unbranched polysaccharide composed of repeating units of glucose.
(i) Draw the structure of the repeating unit of amylose. Use section 34 of the data booklet.
(ii) Amylose is a major component of starch. Corn starch can be used to make replacements for plastics derived from oil, especially for packaging. Discuss one potential advantage and one disadvantage of this use of starch.
- 16N.3.sl.TZ0.17a: Zanamivir must be taken by inhalation, not orally. Deduce what this suggests about the...
-
16N.3.hl.TZ0.16c:
A student investigated the ability of anthocyanins to act as pH indicators. He extracted juice from blackberries and used a UV-vis spectrophotometer to produce absorption spectra at different pH values. His results are shown below.
Deduce the colour of the juice at each pH, giving your reasoning. Use section 17 of the data booklet.
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
-
16N.3.hl.TZ0.29b:
One class of performance-enhancing drugs is the anabolic steroids. Detection of these drugs in urine samples uses a combination of gas chromatography and mass spectrometry (GC/MS).
(i) Describe how gas chromatography enables the components of urine to be analysed.
(ii) The structures of two steroids, testosterone and nandrolone, are given below.
With reference to the molar masses of the two steroids, determine, with a reason, which can be identified from the mass spectrum below.
-
16N.3.sl.TZ0.5b:
Nanocatalysts play an essential role in the manufacture of industrial chemicals.
(i) Describe the high pressure carbon monoxide (HIPCO) method for the production of carbon nanotubes.
(ii) Outline one benefit of using nanocatalysts compared to traditional catalysts in industry.
-
16N.3.sl.TZ0.8b:
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
-
16N.3.sl.TZ0.16a:
(i) Outline what is meant by the term “ring strain”.
(ii) On the diagram above, label with asterisk/s (*) the carbon atom/s that experience ring strain.
- 16N.3.sl.TZ0.12a: Discuss how the octane number changes with the molecular structure of the alkanes.
- 16N.3.hl.TZ0.9a: Describe the Meissner effect.
-
16N.3.hl.TZ0.15b:
In 2010, scientists claimed that they had discovered a species of bacteria capable of incorporating arsenic in place of phosphorus into the bacterial DNA. This claim has since proved controversial. Suggest one technique or evidence that might help support the claim.
- 16N.3.hl.TZ0.28c: Outline two reasons, other than its half-life, why technetium-99m is so useful in medical diagnosis.
- 16N.3.sl.TZ0.3a: Magnesium oxide, MgO, and silicon carbide, SiC, are examples of ceramic materials. State the name...
-
16N.3.sl.TZ0.4a:
Calculate the charge, in coulombs, passed during the electrolysis.
-
16N.3.sl.TZ0.4b:
Calculate the amount, in mol, of electrons passed using section 2 of the data booklet.
-
16N.3.sl.TZ0.4c:
Calculate the mass of indium deposited by one mole of electrons.
-
16N.3.sl.TZ0.4d:
Calculate the number of moles of electrons required to deposit one mole of indium. Relative atomic mass of indium, Ar=114.82.
- 16N.3.sl.TZ0.5a: Explain, with reference to their structure, the great selectivity of zeolites as catalysts.
- 16N.3.sl.TZ0.7a: Outline how a lyotropic liquid crystal differs from a thermotropic liquid crystal.
- 16N.3.sl.TZ0.9a: State the raw materials and source of energy used in the process described above.
- 16N.3.sl.TZ0.10c: Determine the number of different tripeptides that can be made from twenty different amino acids.
-
16N.3.sl.TZ0.11b:
(i) The energy density of gasoline is 34.3 MJ dm−3. Calculate the volume of gasoline, in dm3, that is equivalent to the energy in 80.0 kg of fuel in the lithium ion battery. Use section 1 of the data booklet.
(ii) The efficiency of energy transfer by this lithium ion battery is four times greater than that of gasoline. Determine the distance, in km, the car can travel on the lithium ion battery power alone if the gasoline-powered car uses 1.00 dm3 gasoline to travel 32.0 km.
- 16N.3.sl.TZ0.12b: Catalytic reforming and cracking reactions are used to produce more efficient fuels. Deduce the...
-
16N.3.sl.TZ0.14b:
Outline why the fuel produced by the reaction in (a) is more suitable for use in diesel engines than vegetable oils.
-
16N.3.sl.TZ0.15a:
(i) Explain why fusion, combining two smaller nuclei into a larger nucleus, releases vast amounts of energy. Use section 36 of the data booklet.
(ii) Outline one advantage of fusion as a source of energy.
-
16N.3.sl.TZ0.16b:
(i) Some antibiotic-resistant bacteria produce a beta-lactamase enzyme which destroys penicillin activity. Suggest how adding clavulanic acid to penicillin enables the antibiotic to retain its activity.
(ii) Populations of antibiotic-resistant bacteria have increased significantly over the last 60 years. Outline why antibiotics such as penicillin should not be prescribed to people suffering from a viral infection.
- 16N.3.sl.TZ0.17c: The synthesis of oseltamivir is dependent on a supply of the precursor shikimic acid, which is...
- 16N.3.sl.TZ0.18d: State why aspirin is described as a mild analgesic with reference to its site of action.
-
16N.3.sl.TZ0.19a:
Ranitidine (Zantac) is a drug that inhibits stomach acid production. Outline why the development of this drug was based on a detailed knowledge of the structure of histamine, shown below.
-
16N.3.hl.TZ0.6d:
Fermentation of sugars from corn starch produces propane-1,3-diol, which can be polymerized with benzene-1,4-dicarboxylic acid to produce the PTT polymer (polytrimethylene terephthalate).
(i) Draw the molecular structure of each monomer.
(ii) Deduce the name of the linkage formed on polymerization between the two monomers and the name of the inorganic product.
- 16N.3.sl.TZ0.3b: Predict the predominant type of bonding for a binary compound AB in which the electronegativity...
-
16N.3.sl.TZ0.6b:
Deduce the percentage atom economy for polymerization of 2-methylpropene.
-
16N.3.sl.TZ0.19b:
Two other drugs, omeprazole (Prilosec) and esomeprazole (Nexium), directly prevent the release of acid into the stomach. Identify the site of action in the body.
-
16N.3.hl.TZ0.14b:
(i) Outline what is meant by product inhibition as it applies to hexokinase.
(ii) Product inhibition of hexokinase does not affect its Km value. Using this information, deduce the type of binding site that the inhibitor attaches to.
- 16N.3.hl.TZ0.15a: State the name of the component of DNA responsible for the migration of its fragments to the...
- 16N.3.hl.TZ0.16a: Outline why this molecule absorbs visible light.
-
16N.3.hl.TZ0.8b:
(i) The mass of one unit cell of chromium metal is 17.28 × 10−23 g. Calculate the number of unit cells in one mole of chromium. Ar(Cr) = 52.00.
(ii) Deduce the number of atoms of chromium per unit cell.
-
16N.3.hl.TZ0.14a:
(i) Estimate the Km values of the two enzymes.
(ii) Suggest, with a reason, which enzyme will be more responsive to changes in the concentration of glucose in the blood.
- 16N.3.hl.TZ0.16b: With reference to its chemical structure, outline whether this pigment is found in aqueous...
- 16N.3.hl.TZ0.29a: Suggest what may have led to these changes in acceptable concentrations.
- 20N.3.sl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.sl.TZ0.3c: Explain why phthalates are replaced by other plasticizers in the production of plastics.
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
- 20N.3.sl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
- 20N.3.sl.TZ0.4d: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
-
20N.3.sl.TZ0.5b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
- 20N.3.sl.TZ0.4a: Explain these properties of carbon nanotubes.
-
20N.3.sl.TZ0.4b(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020.
Write the half-equations for the reactions occurring in this electrolysis.
-
20N.3.sl.TZ0.3a:
Outline the two distinct phases of this composite.
-
20N.3.sl.TZ0.4b(iv):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
- 20N.3.sl.TZ0.4c: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
-
20N.3.sl.TZ0.6a:
Deduce the products of the hydrolysis of a non-substituted phospholipid, where and represent long alkyl chains.
- 20N.3.sl.TZ0.7a: The diverse functions of biological molecules depend on their structure and shape. Classify...
-
20N.3.sl.TZ0.7b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
-
20N.3.sl.TZ0.8a:
Calculate the BMF if a shark consumes mackerel in one year. Each mackerel weighs on average. The per body weight. Assume chemical remains in the shark’s body for two years.
-
20N.3.sl.TZ0.4b(iii):
Calculate the theoretical mass of magnesium obtained if a current of is used for hours. Use charge and section 2 of the data booklet
-
20N.3.sl.TZ0.7b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Sucrose is a disaccharide formed in the reaction of glucose with fructose.
Identify the reaction type and the newly formed functional group that joins the monosaccharide units in the product.
-
20N.3.sl.TZ0.6c:
Phospholipids help maintain cellular environments while fatty acid lipids have important roles in energy storage and electrical insulation. Discuss the structural properties of saturated fats needed for these roles.
- 20N.3.sl.TZ0.8b: Suggest, with a reason, if fat-soluble or water-soluble xenobiotics would have a larger BMF.
- 20N.3.sl.TZ0.9e: Contrast the molecular structures of biodiesel and the vegetable oil from which it is formed.
-
20N.3.sl.TZ0.6b(i):
A representation of a phospholipid bilayer cell membrane is shown:
© International Baccalaureate Organization 2020.
Identify the components of the phospholipid labelled A and B.
-
20N.3.sl.TZ0.5a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.sl.TZ0.5c:
Proteins are polymers of amino acids.
Describe how the tertiary structure differs from the quaternary structure in hemoglobin.
-
20N.3.sl.TZ0.5a(i):
Proteins are polymers of amino acids. A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
-
20N.3.sl.TZ0.9a:
Calculate the energy released, in , from the complete combustion of of ethanol.
-
20N.3.sl.TZ0.9d:
A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
-
20N.3.sl.TZ0.9c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
-
20N.3.sl.TZ0.9f(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
-
20N.3.sl.TZ0.11d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
- 20N.3.sl.TZ0.10e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
-
20N.3.sl.TZ0.11a:
Deduce the structural formula of the by-product of this reaction.
-
20N.3.sl.TZ0.14b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
- 20N.3.sl.TZ0.9b: State a class of organic compounds found in gasoline.
-
20N.3.sl.TZ0.13b:
Outline a green chemistry solution for problems generated by the use of organic solvents.
- 20N.3.sl.TZ0.14b(i): Circle the side-chain in penicillin on the structure below.
-
20N.3.sl.TZ0.10b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
- 20N.3.sl.TZ0.13a: Describe the proper disposal of low-level radioactive waste in hospitals.
-
20N.3.sl.TZ0.11c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
- 20N.3.sl.TZ0.10c: Outline a health risk produced by exposure to radioactive decay.
- 20N.3.sl.TZ0.14c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
-
20N.3.sl.TZ0.10d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
- 20N.3.sl.TZ0.14c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
-
20N.3.sl.TZ0.9f(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
- 20N.3.sl.TZ0.14a(i): Name two functional groups that both zanamivir and oseltamivir contain.
- 20N.3.sl.TZ0.11b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.sl.TZ0.14a(ii):
Explain how zanamivir works as a preventative agent against flu viruses.
-
20N.3.sl.TZ0.12:
Consider the following antacids:
Show that antacid X is more effective, per tablet, than antacid Y.
-
20N.3.hl.TZ0.6a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.hl.TZ0.6b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
-
20N.3.hl.TZ0.10b(ii):
Outline the significance of the value of the Michaelis constant, .
- 20N.3.hl.TZ0.14b: Doping of silicon increases the conductivity in semiconductors. Explain how doping improves the...
- 20N.3.hl.TZ0.3b(iii): Explain why phthalates are replaced by other plasticizers in the production of plastics.
- 20N.3.hl.TZ0.4d: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
- 20N.3.hl.TZ0.10a: Identify the type of inhibition shown in the graph.
-
20N.3.hl.TZ0.8b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Draw the nitrogenous base that is paired with guanine in DNA, showing the hydrogen bonds between the bases. Use section 34 of the data booklet.
-
20N.3.hl.TZ0.11a:
Calculate the energy released, in , from the complete combustion of of ethanol.
- 20N.3.hl.TZ0.11d: A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture...
- 20N.3.hl.TZ0.12e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
-
20N.3.hl.TZ0.6c(ii):
Proteins are polymers of amino acids.
Explain why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin.
-
20N.3.hl.TZ0.3a:
Outline the two distinct phases of this composite.
- 20N.3.hl.TZ0.4b(i): CNT can act as Type 2 superconductors. Outline why Type 2 superconductors are generally more...
-
20N.3.hl.TZ0.11e(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
- 20N.3.hl.TZ0.4a: Explain these properties of carbon nanotubes.
-
20N.3.hl.TZ0.5a:
Precipitation is one method used to treat waste water.
Phosphates, , in waste water can be removed by precipitation with magnesium ions. of magnesium phosphate is .
Calculate the maximum solubility of phosphate ions in a solution containing magnesium ions.
- 20N.3.hl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.hl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
-
20N.3.hl.TZ0.4c(iii):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
- 20N.3.hl.TZ0.11b: State a class of organic compounds found in gasoline.
-
20N.3.hl.TZ0.6a(i):
Proteins are polymers of amino acids.
A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
- 20N.3.hl.TZ0.6c(i): Proteins are polymers of amino acids. Sketch and label two oxygen dissociation curves, one for...
-
20N.3.hl.TZ0.4b(ii):
Explain the role of electrons in superconducting materials in terms of the Bardeen–Cooper–Schrieffer (BCS) theory.
-
20N.3.hl.TZ0.12f:
Determine the nuclear binding energy, in , of using sections 2 and 4 of the data booklet.
The mass of the nucleus is .
- 20N.3.hl.TZ0.3c: Classify PVC and polyethene terephthalate, PET, as addition or condensation polymers and deduce...
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
- 20N.3.hl.TZ0.12c: Outline a health risk produced by exposure to radioactive decay.
-
20N.3.hl.TZ0.8b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
- 20N.3.hl.TZ0.8c: The diverse functions of biological molecules depend on their structure and shape. Retinal is...
- 20N.3.hl.TZ0.8a: The diverse functions of biological molecules depend on their structure and shape. Classify...
-
20N.3.hl.TZ0.10b(i):
Determine the value of and in the absence and presence of the inhibitor.
-
20N.3.hl.TZ0.11e(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
-
20N.3.hl.TZ0.12b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
-
20N.3.hl.TZ0.12d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
-
20N.3.hl.TZ0.11c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
-
20N.3.hl.TZ0.11e(iv):
Determine the relative rate of effusion of methane () to carbon dioxide (), under the same conditions of temperature and pressure. Use section 1 of the data booklet.
- 20N.3.hl.TZ0.17b: Discuss the properties that make a radioisotope suitable for diagnosis.
- 20N.3.hl.TZ0.15b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.hl.TZ0.15c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
-
20N.3.hl.TZ0.17c:
Describe the proper disposal of low-level radioactive waste in hospitals.
-
20N.3.hl.TZ0.15e:
Explain how IR spectroscopy can be used to distinguish aspirin from salicylic acid.
-
20N.3.hl.TZ0.15d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
-
20N.3.hl.TZ0.17a:
State the type of radiation technetium-99m emits.
-
20N.3.hl.TZ0.14a:
Doping of silicon increases the conductivity in semiconductors.
Describe the doping in p-type and n-type semiconductors.
-
20N.3.hl.TZ0.15a:
Deduce the structural formula of the by-product of this reaction.
-
20N.3.hl.TZ0.17d:
Technetium-99m has a half-life of hours. Calculate the amount of of technetium-99m remaining after hours.
-
20N.3.hl.TZ0.18a(ii):
The vapour pressure of pure ethanal at is .
Calculate the vapour pressure of ethanal above the liquid mixture at .
-
20N.3.hl.TZ0.19a:
Explain how zanamivir works as a preventative agent against flu viruses.
-
20N.3.hl.TZ0.5b:
Precipitation is one method used to treat waste water.
Zinc, cadmium, nickel, and lead are metal ions which can be removed by precipitation. Explain why waste water is adjusted to a pH of 9−10 to remove these ions by referring to section 32 of the data booklet.
- 20N.3.hl.TZ0.19c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
- 20N.3.hl.TZ0.18b: Describe how this mixture is separated by fractional distillation.
- 20N.3.hl.TZ0.19c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
-
20N.3.hl.TZ0.4c(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020
Calculate the theoretical mass of magnesium obtained if a current of 3.00 A is used for hours. Use charge :(Q) = current (I) × time (t) and section 2 of the data booklet.
- 20N.3.hl.TZ0.19b(i): Circle the side-chain in penicillin on the structure below.
-
20N.3.hl.TZ0.19b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
- 20N.3.hl.TZ0.19d: Circle two chiral carbons in the section of the Taxol structure below.
- 20N.3.hl.TZ0.4e: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
-
17M.3.sl.TZ1.6a:
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
-
17M.3.sl.TZ1.6b:
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
-
17M.3.sl.TZ1.7a:
State why lanthanum cannot be produced by reducing its oxide with carbon.
-
17M.3.sl.TZ1.7b:
Calculate the current (I), in A, required to produce 1.00 kg of lanthanum metal per hour. Use the formula and sections 2 and 6 of the data booklet.
-
17M.3.sl.TZ1.8a:
State the major advantage that nanoparticles have in these applications.
-
17M.3.sl.TZ1.8b:
Suggest why nanoparticles need to be handled with care.
-
17M.3.sl.TZ1.9a:
Catalysts reduce the activation energy. Outline how homogeneous catalysts are involved in the reaction mechanism.
-
17M.3.sl.TZ1.9b:
Suggest why it is important to know how catalysts function.
-
17M.3.sl.TZ1.9c:
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.sl.TZ1.10a:
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
-
17M.3.sl.TZ1.10b.i:
Describe the difference in their structures.
-
17M.3.sl.TZ1.10b.ii:
Explain why the difference in their structures affects their melting points.
-
17M.3.sl.TZ1.11a:
List the building blocks of triglycerides and carbohydrates.
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ1.11b.ii:
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
-
17M.3.sl.TZ1.11c:
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16. -
17M.3.sl.TZ1.12a.i:
Deduce the straight chain structure of ribose from its ring structure drawn in section 34 of the data booklet.
-
17M.3.sl.TZ1.12a.ii:
Using the partial structure given, complete the structural formula of the molecule formed from the condensation of two cyclic -glucose molecules.
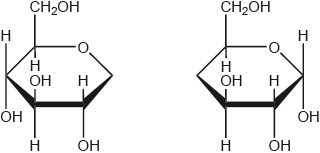
-
17M.3.sl.TZ1.12b:
Constructing models that allow visualizations of the stereochemistry of carbohydrates is essential to understand their structural roles in cells.
Describe how Haworth projections help focus on the position of attached groups.
-
17M.3.sl.TZ1.12c.i:
State one advantage of starch based polymers besides being biodegradable.
-
17M.3.sl.TZ1.12c.ii:
Biodegradable boxes made from polylactic acid, PLA, disintegrate when exposed to water.
State the formula of the product formed when water reacts with PLA.
-
17M.3.sl.TZ1.13a:
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
-
17M.3.sl.TZ1.13b.i:
Identify the name of the amino acid that does not move under the influence of the applied voltage.
-
17M.3.sl.TZ1.13b.ii:
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
-
17M.3.sl.TZ1.13c:
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
-
17M.3.sl.TZ1.13d:
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
-
17M.3.sl.TZ1.13e:
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
-
17M.3.sl.TZ1.13f:
Outline how lead ions could be removed from an individual suffering from lead poisoning.
-
17M.3.sl.TZ1.14a:
Outline how the spectra of light from stars can be used to detect the presence of carbon.
-
17M.3.sl.TZ1.14b.i:
Deduce the identity of X.
-
17M.3.sl.TZ1.14b.ii:
Outline why this reaction results in a release of energy.
-
17M.3.sl.TZ1.14c:
Nuclear fusion reactors are predicted to become an important source of electrical energy in the future. State two advantages of nuclear fusion over nuclear fission.
-
17M.3.sl.TZ1.15a:
State two reagents required to convert vegetable oil to biodiesel.
-
17M.3.sl.TZ1.15b:
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
-
17M.3.sl.TZ1.15c:
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
-
17M.3.sl.TZ1.15d:
Determine the specific energy, in kJg−1, and energy density, in kJcm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 gcm−3; Molar mass = 299 gmol−1;
Enthalpy of combustion = 12.0 MJmol−1.
-
17M.3.sl.TZ1.16a:
State how these gases are produced, giving the appropriate equation(s).
-
17M.3.sl.TZ1.16b:
Outline how the carbon monoxide is then converted to a hydrocarbon fuel.
-
17M.3.sl.TZ1.17a:
Suggest why it is only in recent years that specific predictions of the future effects of fossil fuel combustion have been made.
-
17M.3.sl.TZ1.17b:
Carbon dioxide has two different bond stretching modes illustrated below.

Predict, with an explanation, whether these stretching modes will absorb infrared radiation.
-
17M.3.sl.TZ1.17c:
Outline, giving the appropriate equation(s), how increasing levels of carbon dioxide will affect the pH of the oceans.
-
17M.3.sl.TZ1.17d:
Many combustion processes also release particulate matter into the atmosphere. Suggest, giving your reason, how this might affect the temperature of the Earth’s surface.
-
17M.3.sl.TZ1.18a:
Dose response curves are determined for each drug.
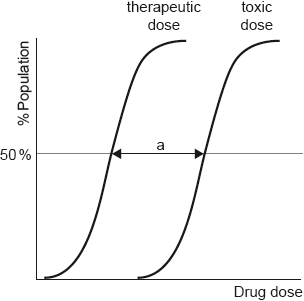
Outline the significance of range “a”.
-
17M.3.sl.TZ1.18b.i:
Suggest the type of reaction used to convert morphine to codeine.
-
17M.3.sl.TZ1.18b.ii:
State and explain the action of opiates as painkillers.
-
17M.3.sl.TZ1.19a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.sl.TZ1.19b:
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
-
17M.3.sl.TZ1.19c:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
17M.3.sl.TZ1.19d:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
17M.3.sl.TZ1.19e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
17M.3.sl.TZ1.20a:
Calculate the pH of the buffer from the following data and section 1 of the data booklet.
pKa(CO2) = 6.34
[HCO3−(aq)] = 1.40 × 10−2 moldm−3
[CO2(aq)] = 1.25 × 10−3 moldm−3
-
17M.3.sl.TZ1.20b:
Explain the effect of a large amount of aspirin on the pH of blood.
-
17M.3.sl.TZ1.21a:
Outline how oseltamivir (Tamiflu®) works.
-
17M.3.sl.TZ1.21b:
Oseltamivir was commercially produced from shikimic acid, a precursor which is a metabolite in micro-organisms and plants.
Outline how green chemistry was used to develop the precursor for oseltamivir in order to overcome a shortage of the drug during the flu season.
-
17M.3.sl.TZ1.21c:
Suggest why the administration of antibiotics to humans and animals can affect the environment.
-
17M.3.hl.TZ1.8a:
Lanthanum has a hexagonal close packed (hcp) crystal structure. State the coordination number of each lanthanum atom.
-
17M.3.hl.TZ1.8b:
Lanthanum becomes superconducting below 5 K. Explain, in terms of Bardeen–Cooper–Schrieffer (BCS) theory, how superconductivity occurs.
-
17M.3.hl.TZ1.8c:
Outline why superconductivity only occurs at low temperatures.
-
17M.3.hl.TZ1.9a:
Deduce the repeating unit of the polymer and the other product of the reaction.
-
17M.3.hl.TZ1.9b:
State the class of polymer to which PETE belongs.
-
17M.3.hl.TZ1.10a:
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.hl.TZ1.10b:
Hydrogen sulfide could be used to remove antimony(III) ions from a solution.
Determine the concentration of antimony(III) ions that would be required to precipitate antimony(III) sulfide in a solution saturated with hydrogen sulfide.
[S2−] in water saturated with hydrogen sulfide = 1.0 × 10−14 mol dm−3
Ksp (Sb2S3) = 1.6 × 10−93
-
17M.3.hl.TZ1.10c:
Identify a ligand that could be used to chelate antimony(III) ions in solution.
-
17M.3.hl.TZ1.15a:
Deduce the pH range in which glycine is an effective buffer in basic solution.
-
17M.3.hl.TZ1.15b:
Enzymes are biological catalysts.
The data shows the effect of substrate concentration, [S], on the rate, v, of an enzyme-catalysed reaction.
Determine the value of the Michaelis constant (Km) from the data. A graph is not required.
-
17M.3.hl.TZ1.15c:
Outline the action of a non-competitive inhibitor on the enzyme-catalysed reaction.
-
17M.3.hl.TZ1.15d:
The sequence of nitrogenous bases in DNA determines hereditary characteristics.
Calculate the mole percentages of cytosine, guanine and thymine in a double helical DNA structure if it contains 17% adenine by mole.
-
17M.3.hl.TZ1.16a:
Explain the shape of the curve at low oxygen partial pressure up to about 5 kPa.
-
17M.3.hl.TZ1.16b.i:
Sketch a graph on the axes above to show the effect of decreasing pH on the binding of oxygen to hemoglobin (the Bohr Effect).
-
17M.3.hl.TZ1.16b.ii:
Outline the effect of decreasing pH on the oxygen saturation of hemoglobin.
-
17M.3.hl.TZ1.17:
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
-
17M.3.hl.TZ1.18b.ii:
The mass of X is 8.005305 amu and that of is 4.002603 amu. Determine the energy produced, in J, when one atom of is formed in this reaction. Use section 2 of the data booklet.
-
17M.3.hl.TZ1.19a:
Identify two ways in which the structure of the dye shown resembles the chlorophyll molecule. Use section 35 of the data booklet.
-
17M.3.hl.TZ1.19b:
Both photosynthesis and the Grätzel cell use energy from sunlight to bring about reduction. Deduce an equation for the reduction reaction in the electrolyte of a Grätzel cell.
-
17M.3.hl.TZ1.22a:
Deduce half-equations for the reactions at the two electrodes and hence the equation for the overall reaction.
-
17M.3.hl.TZ1.22b.i:
Suggest a way in which they are similar.
-
17M.3.hl.TZ1.22b.ii:
Outline the difference between primary and rechargeable cells.
-
17M.3.hl.TZ1.22c:
Identify one factor that affects the voltage of a cell and a different factor that affects the current it can deliver.
-
17M.3.hl.TZ1.25d:
Some mild analgesics contain a solid mixture of acidic aspirin and a non-acidic organic chemical of similar polarity to asprin.
Discuss how acid-base properties and the process of solvent extraction can be used to separate aspirin from the mixture.
-
17M.3.hl.TZ1.28b:
A mixture of enantiomers shows optical rotation.
Suggest a conclusion you can draw from this data.
-
17M.3.hl.TZ1.29a:
Yttrium-90 is used in treating certain cancers.
Formulate a nuclear equation for the beta decay of yttrium-90.
-
17M.3.hl.TZ1.29b:
Lutetium-177 is a common isotope used for internal radiation therapy.
Suggest why lutetium-177 is an ideal isotope for the treatment of certain cancers based on the type of radiation emitted.
-
17M.3.hl.TZ1.29c.i:
Calculate the rate constant, , in day−1, for the decay of iodine-131 using section 1 of the data booklet.
-
17M.3.hl.TZ1.29c.ii:
Calculate the time, in days, for 90% of the sample to decay.
-
17M.3.hl.TZ1.29d:
A breathalyser measures the blood alcohol content from a breath sample. Formulate half-equations for the reactions at the anode (negative electrode) and the cathode (positive electrode) in a fuel cell breathalyser.
-
17M.3.sl.TZ2.3a:
State the two distinct phases of a composite.
-
17M.3.sl.TZ2.3b:
Identify the methods of assembling nanocomposites by completing the table.
-
17M.3.sl.TZ2.3c.i:
Explain how the structure of plasticizers enables them to soften PVC.
-
17M.3.sl.TZ2.3c.ii:
Suggest a reason why nanoparticles can better anchor plasticizers in the polymer.
-
17M.3.sl.TZ2.5a:
In a catalytic converter, carbon monoxide is converted to carbon dioxide. Outline the process for this conversion referring to the metal used.
-
17M.3.sl.TZ2.5b.i:
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
-
17M.3.sl.TZ2.5c:
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
-
17M.3.sl.TZ2.6a:
Two important properties of a liquid crystal molecule are being a polar molecule and having a long alkyl chain. Explain why these are essential components of a liquid crystal molecule.
-
17M.3.sl.TZ2.6b:
Metal impurities during the production of LCoS can be analysed using ICP-MS. Each metal has a detection limit below which the uncertainty of data is too high to be valid. Suggest one factor which might influence a detection limit in ICP-MS/ICP-OES.
-
17M.3.sl.TZ2.7a:
Deduce the structural formula of the dipeptide Cys-Lys.
-
17M.3.sl.TZ2.7b:
Identify the type of bond between two cysteine residues in the tertiary structure of a protein.
-
17M.3.sl.TZ2.7c:
Deduce the structural formula of the predominant form of cysteine at pH 1.0.
-
17M.3.sl.TZ2.7d:
A mixture of the three amino acids, cysteine, glutamine and lysine, was placed in the centre of a square plate covered in polyacrylamide gel. The gel was saturated with a buffer solution of pH 6.0. Electrodes were connected to opposite sides of the gel and a potential difference was applied.
Sketch lines on the diagram to show the relative positions of the three amino acids after electrophoresis.
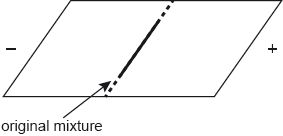
-
17M.3.sl.TZ2.8a:
Explain which one of these fatty acids has the highest boiling point.
-
17M.3.sl.TZ2.8b:
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 moldm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
-
17M.3.sl.TZ2.9a:
Glycerol is one product of the reaction. Identify the two other organic products.
-
17M.3.sl.TZ2.9b:
Identify the type of reaction which occurs.
-
17M.3.sl.TZ2.10a:
Identify the functional groups which are present in only one structure of glucose.
-
17M.3.sl.TZ2.10b:
Sucrose is a disaccharide formed from -glucose and β-fructose.
Deduce the structural formula of sucrose.
-
17M.3.sl.TZ2.10c:
Starch is a constituent of many plastics. Suggest one reason for including starch in plastics.
-
17M.3.sl.TZ2.10d:
Suggest one of the challenges scientists face when scaling up the synthesis of a new compound.
-
17M.3.sl.TZ2.11:
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
-
17M.3.sl.TZ2.12b:
Coloured molecules absorb sunlight. Identify the bonding characteristics of such molecules.
-
17M.3.sl.TZ2.13a:
State one advantage and one disadvantage for each energy source in the table.
-
17M.3.sl.TZ2.13b.i:
Calculate the specific energy of hydrogen, stating its units. Refer to sections 1, 6 and 13 of the data booklet.
-
17M.3.sl.TZ2.13b.ii:
Hydrogen has a higher specific energy than petrol (gasoline) but is not used as a primary fuel source in cars. Discuss the disadvantages of using hydrogen.
-
17M.3.sl.TZ2.14a:
Identify which region, A or B, corresponds to each type of radiation by completing the table.
-
17M.3.sl.TZ2.14b.i:
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
-
17M.3.sl.TZ2.14b.ii:
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
-
17M.3.sl.TZ2.14c.i:
Suggest an equation for the production of syngas from coal.
-
17M.3.sl.TZ2.14c.ii:
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
-
17M.3.sl.TZ2.14c.iii:
Suggest a reason why syngas may be considered a viable alternative to crude oil.
-
17M.3.sl.TZ2.15a.iii:
State two techniques which could be used to confirm the identity of aspirin.
-
17M.3.sl.TZ2.15b.i:
State how aspirin can be converted to water-soluble aspirin.
-
17M.3.sl.TZ2.15b.ii:
Compare, giving a reason, the bioavailability of soluble aspirin with aspirin.
-
17M.3.sl.TZ2.16a:
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
-
17M.3.sl.TZ2.16b:
Suggest a reagent used to prepare diamorphine from morphine.
-
17M.3.sl.TZ2.16c:
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
-
17M.3.sl.TZ2.17a:
Two drugs are ranitidine (Zantac) and omeprazole (Prilosec). Outline how they function to reduce stomach acidity.
-
17M.3.sl.TZ2.17b:
0.500 g of solid anhydrous sodium carbonate, Na2CO3(s), is dissolved in 75.0 cm3 of 0.100 moldm−3 sodium hydrogen carbonate solution, NaHCO3(aq). Assume the volume does not change when the salt dissolves.
HCO3−(aq) CO32−(aq) + H+(aq) pKa = 10.35.
Calculate the pH of the buffer solution.
-
17M.3.sl.TZ2.18a.i:
Compare and contrast the structures of oseltamivir and zanamivir, stating the names of functional groups.
-
17M.3.sl.TZ2.18a.ii:
Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds, using section 26 of the data booklet.
-
17M.3.sl.TZ2.18b:
Suggest one ethical consideration faced by medical researchers when developing medications.
-
17M.3.sl.TZ2.19a:
Suggest one problem associated with chlorinated organic solvents as chemical waste.
-
17M.3.sl.TZ2.19b:
Suggest how the principles of green chemistry can be used to solve the environmental problems caused by organic solvents.
-
17M.3.hl.TZ2.3c:
Estimate the atom economy of this first step.
-
17M.3.hl.TZ2.3c.ii:
Suggest, giving one reason, whether this is an addition or condensation reaction.
-
17M.3.hl.TZ2.4a:
Identify the other product formed.
-
17M.3.hl.TZ2.4b:
Explain why EDTA, a chelating agent, is more effective in removing heavy metal ions from solution than monodentate ligands.
-
17M.3.hl.TZ2.5b.iii:
Nickel(II) ions are least soluble at pH 10.5. Calculate the molar solubility of nickel(II) hydroxide at this pH. KspNi(OH)2 = 5.48 × 10–16.
-
17M.3.hl.TZ2.5c.i:
Rhodium is paramagnetic with an electron configuration of [Kr] 5s14d8.
Explain, in terms of electron spin pairing, why paramagnetic substances are attracted to a magnetic field and diamagnetic substances are not.
-
17M.3.hl.TZ2.5c.ii:
Rhodium is a type 1 superconductor.
Sketch graphs of resistance against temperature for a conductor and superconductor.
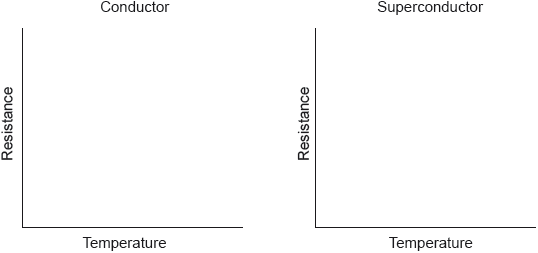
-
17M.3.hl.TZ2.5c.iii:
Contrast type 1 and type 2 superconductors by referring to three differences between them.
-
17M.3.hl.TZ2.8c.i:
An aqueous buffer solution contains both the zwitterion and the anionic forms of alanine. Draw the zwitterion of alanine.
-
17M.3.hl.TZ2.8c.ii:
Calculate the pH of a buffer solution which contains 0.700 mol dm–3 of the zwitterion and 0.500 mol dm–3 of the anionic form of alanine.
Alanine pKa = 9.87.
-
17M.3.hl.TZ2.12a:
Identify the structural feature which enables rhodopsin to absorb visible light.
-
17M.3.hl.TZ2.12b:
Outline the change that occurs in the retinal residue during the absorption of visible light.
-
17M.3.hl.TZ2.13a:
Determine the value of the Michaelis constant, Km, including units, from the graph.
-
17M.3.hl.TZ2.13b:
Sketch a second graph on the same axes to show how the reaction rate varies when a competitive inhibitor is present.
-
17M.3.hl.TZ2.13c:
Outline the significance of the value of Km.
-
17M.3.hl.TZ2.14a:
Explain the shape of the curve from 0 to X kPa.
-
17M.3.hl.TZ2.14b:
Explain why carbon monoxide is toxic to humans.
-
17M.3.hl.TZ2.15a:
Outline how its structure allows it to be negatively charged in the body.
-
17M.3.hl.TZ2.15b:
Deduce the nucleotide sequence of a complementary strand of a fragment of DNA with the nucleotide sequence –GACGGATCA–.
-
17M.3.sl.TZ2.12a.i:
One fusion reaction occurring in the sun is the fusion of deuterium, , with tritium, , to form helium, . State a nuclear equation for this reaction.
-
17M.3.sl.TZ2.12a.ii:
Explain why this fusion reaction releases energy by using section 36 of the data booklet.
-
17M.3.hl.TZ2.16a.iii:
Calculate the energy released, in MeV, in this reaction, using section 36 of the data booklet.
-
17M.3.hl.TZ2.17c.i:
Deduce the half-cell equations occurring at each electrode during discharge.
-
17M.3.hl.TZ2.17c.ii:
Outline the function of the proton-exchange membrane (PEM) in the fuel cell.
-
17M.3.hl.TZ2.17c.iii:
Explain how the flow of ions allows for the operation of the fuel cell.
-
17M.3.hl.TZ2.18a.ii:
The structures of 11-cis-retinal and β-carotene are given in section 35 of the data booklet. Suggest a possible wavelength of light absorbed by each molecule using section 3 of the data booklet.
-
17M.3.hl.TZ2.19a:
Contrast how absorption of photons and charge separation occur in each device.
-
17M.3.hl.TZ2.19b:
Suggest one advantage a DSSC has over a silicon based photovoltaic cell.
-
17M.3.hl.TZ2.20a.iv:
State two techniques, other than IR spectroscopy, which could be used to confirm the identity of aspirin.
-
17M.3.hl.TZ2.22a:
Outline how ranitidine (Zantac) functions to reduce stomach acidity.
-
17M.3.hl.TZ2.25:
Taxol is produced using a chiral auxiliary. Describe how the chiral auxiliary functions to produce the desired product.
-
17M.3.hl.TZ2.26a.i:
Explain why alpha-radiation is particularly suitable for this treatment.
-
17M.3.hl.TZ2.26a.ii:
Outline how the alpha-radiation in TAT is directed to cancer cells.
-
17M.3.hl.TZ2.26b.i:
Identify the type of radiation emitted by these two radioisotopes.
-
17M.3.hl.TZ2.26b.ii:
State an equation for the one-step decay of yttrium-90.
-
17M.3.hl.TZ2.26b.iii:
The half-life of lutetium-177 is 6.75 days. Calculate the percentage remaining after 27 days.
-
17N.3.hl.TZ0.18c.i:
Calculate the loss in mass, in kg, and the energy released, in J, when 0.00100 mol of 228Ac decays, each atom losing an electron. Use section 2 of the data booklet and E = mc2.
228Ac → + 228Th
-
17N.3.sl.TZ0.4a:
Outline the composition of an alloy and a composite.
-
17N.3.sl.TZ0.6a:
State equations for the formation of iron nanoparticles and carbon atoms from Fe(CO)5 in the HIPCO process.
- 17N.3.sl.TZ0.8a.ii: The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per...
- 17N.3.hl.TZ0.7b: Describe how the monomers of addition polymers and of condensation polymers differ.
-
17N.3.sl.TZ0.8c:
Outline the importance of linoleic acid for human health.
-
17N.3.hl.TZ0.11a:
Determine the value of the Michaelis constant, Km, by annotating the graph.
-
17N.3.hl.TZ0.13:
The stability of DNA is due to interactions of its hydrophilic and hydrophobic components.
Outline the interactions of the phosphate groups in DNA with water and with surrounding proteins (histones).
-
17N.3.hl.TZ0.21c:
Explain the low environmental impact of most medical nuclear waste.
-
17N.3.hl.TZ0.23b:
Explain the role of the chiral auxiliary in the synthesis of Taxol.
-
17N.3.hl.TZ0.9b:
The solubility product, Ksp , of cadmium sulfide, CdS, is 8.0 × 10–27. Determine the concentration of cadmium ions in 1.0 dm3 of a saturated solution of cadmium sulfide to which 0.10 mol of solid sodium sulfide has been added, stating any assumption you make.
- 17N.3.sl.TZ0.21: Molecules of antibiotics often contain a beta-lactam ring. Explain the importance of the...
-
17N.3.hl.TZ0.18c.ii:
Determine the energy released, in J, by 0.00100 mol of 228Ac over the course of 18 hours.
- 17N.3.hl.TZ0.18d: Outline how nuclear ionising radiation can damage DNA and enzymes in living cells.
-
17N.3.hl.TZ0.20b:
Outline one advantage and one disadvantage of the methanol cell (DMFC) compared with a hydrogen-oxygen fuel cell.
-
17N.3.hl.TZ0.8a:
Calculate the total number of cobalt atoms within its unit cell.
-
17N.3.sl.TZ0.12b:
A typical wood has a specific energy of 17 × 103 kJ kg–1. Comment on the usefulness of octane and wood for powering a moving vehicle, using your answer to (a).
If you did not work out an answer for (a), use 45 × 103 kJ kg–1 but this is not the correct answer.
-
17N.3.sl.TZ0.7b.ii:
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
-
17N.3.sl.TZ0.9c:
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
-
17N.3.hl.TZ0.11b.i:
The malonate ion acts as an inhibitor for the enzyme.
Suggest, on the molecular level, how the malonate ion is able to inhibit the enzyme.
-
17N.3.hl.TZ0.11b.ii:
Draw a curve on the graph above showing the effect of the presence of the malonate ion inhibitor on the rate of reaction.
-
17N.3.hl.TZ0.14a:
State the half-equation for the reduction of molecular oxygen to water in acidic conditions.
- 17N.3.hl.TZ0.14b: Outline the change in oxidation state of the iron ions in heme groups that occurs when molecular...
-
17N.3.hl.TZ0.21a:
State a nuclear equation to show the decay of lutetium-177.
-
17N.3.hl.TZ0.15b:
Retinal is the key molecule involved in vision. Explain the roles of cis and trans-retinal in vision and how the isomers are formed in the visual cycle.
-
17N.3.hl.TZ0.19b:
The natural absorption of light by chlorophyll has been copied by those developing dye-sensitized solar cells (DSSCs). Outline how a DSSC works.
-
17N.3.hl.TZ0.20a:
Deduce the half-equations and the overall equation for the reactions taking place in a direct methanol fuel cell (DMFC) under acidic conditions.
-
17N.3.hl.TZ0.21b:
The half-life of lutetium-177 is 6.73 days. Determine the percentage of a sample of lutetium-177 remaining after 14.0 days.
- 17N.3.hl.TZ0.22a.i: Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
-
17N.3.hl.TZ0.22a.ii:
Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section 26 of the data booklet.
- 17N.3.hl.TZ0.22b: Describe how mild analgesics function.
- 17N.3.hl.TZ0.27: Ethanol slows down the reaction time of a driver leading to traffic accidents. Explain how the...
-
17N.3.hl.TZ0.6b:
Explain why Type 2 superconductors are generally more useful than Type 1.
-
17N.3.hl.TZ0.7c:
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
-
17N.3.hl.TZ0.8b.i:
The atomic radius, r, of cobalt is 1.18 × 10–8 cm. Determine the edge length, in cm, of the unit cell, a, using the second diagram.
-
17N.3.hl.TZ0.8b.ii:
Determine a value for the density of cobalt, in g cm–3, using data from sections 2 and 6 of the data booklet and your answers from (a) and (b) (i).
If you did not obtain an answer to (b) (i), use 3.00 × 10–8 cm but this is not the correct answer.
- 17N.3.hl.TZ0.9a: State the name of one method, other than precipitation, of removing heavy metal ions from...
-
17N.3.sl.TZ0.17b.ii:
Explain why opiates are addictive.
- 17N.3.sl.TZ0.17a: Aspirin is a mild analgesic derived from salicylic acid found in willow bark. Describe how mild...
-
17N.3.sl.TZ0.18a:
Outline the difference between the therapeutic index in animal studies and the therapeutic index in humans.
-
17N.3.sl.TZ0.10a:
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
-
17N.3.sl.TZ0.13a:
“Knocking” in an automobile (car) engine can be prevented by increasing the octane number of the fuel. Explain, including an equation with structural formulas, how heptane, C7H16, could be chemically converted to increase its octane number.
-
17N.3.sl.TZ0.16:
Radioisotopes are used to diagnose and treat various diseases. Explain the low environmental impact of most medical nuclear waste.
- 17N.3.sl.TZ0.4b.i: Outline why an alloy is usually harder than its components by referring to its structure.
- 17N.3.sl.TZ0.4b.ii: At present, composite fillings are more expensive than amalgam fillings. Suggest why a patient...
- 17N.3.sl.TZ0.9b: Draw the structure of galactose on the skeleton provided.
- 17N.3.sl.TZ0.10b: State one function of vitamin D in the body.
- 17N.3.sl.TZ0.11: Enzyme activity depends on many factors. Explain how pH change causes loss of activity of an enzyme.
-
17N.3.sl.TZ0.4c:
Explain how Inductively Coupled Plasma (ICP) Spectroscopy could be used to determine the concentration of mercury in a sample of dental filling.
-
17N.3.sl.TZ0.5:
Catalysts can take many forms and are used in many industrial processes.
Suggest two reasons why it might be worth using a more expensive catalyst to increase the rate of a reaction.
-
17N.3.sl.TZ0.9a:
Describe what is meant by a condensation reaction.
- 17N.3.sl.TZ0.13d: Outline how water and carbon dioxide absorb infrared radiation.
-
17N.3.sl.TZ0.19a:
State the names of two functional groups that both compounds contain, using section 37 of the data booklet.
-
17N.3.sl.TZ0.20b:
The pH is maintained in different fluids in the body by the use of buffers.
Calculate the pH of a buffer solution of 0.0200 mol dm–3 carbonic acid, H2CO3, and 0.400 mol dm–3 sodium hydrogen carbonate, NaHCO3. The pKa of carbonic acid is 6.35.
-
17N.3.sl.TZ0.7a:
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
-
17N.3.sl.TZ0.7c:
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
-
17N.3.sl.TZ0.13b:
Many like to refer to our “carbon footprint”. Outline one difficulty in quantifying such a concept.
-
17N.3.sl.TZ0.14b:
The amount of 228Ac in a sample decreases to one eighth of its original value in about 18 hours due to β-decay. Estimate the half-life of 228Ac.
- 17N.3.sl.TZ0.15a: State the structural feature of chlorophyll that enables it to absorb visible light.
- 17N.3.sl.TZ0.18b: State the method of drug administration that gives the maximum bioavailability.
- 17N.3.sl.TZ0.6c: Discuss one possible risk associated with the use of nanotechnology.
-
17N.3.sl.TZ0.12a:
Calculate the specific energy of octane, C8H18, in kJ kg–1 using sections 1, 6 and 13 of the data booklet.
-
17N.3.sl.TZ0.13c:
Climate change or global warming is a consequence of increased levels of carbon dioxide in the atmosphere. Explain how the greenhouse effect warms the surface of the earth.
-
17N.3.sl.TZ0.14a.i:
Compare and contrast fission and fusion in terms of binding energy and the types of nuclei involved.
-
17N.3.sl.TZ0.14a.ii:
Suggest two advantages that fusion has over fission.
- 17N.3.sl.TZ0.19b: Explain how oseltamivir and zanamivir can stop the spread of the flu virus in the body.
-
17N.3.sl.TZ0.8b.ii:
Calculate the volume of iodine solution used to reach the end-point.
-
17N.3.sl.TZ0.12c:
State the name of one renewable source of energy other than wood.
- 17N.3.sl.TZ0.15b: Vegetable oils are too viscous for use as liquid fuels. Describe, using an equation, how a...
-
17N.3.sl.TZ0.17b.i:
The strong analgesics morphine and codeine are opiates. Outline how codeine can be synthesized from morphine. The structures of morphine and codeine are in section 37 of the data booklet.
-
17N.3.sl.TZ0.20a:
Explain how ranitidine (Zantac) reduces stomach acid production.
- 17N.3.sl.TZ0.6b: Outline why the iron nanoparticle catalysts produced by the HIPCO process are more efficient than...
-
17N.3.sl.TZ0.7b.i:
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
- 17N.3.sl.TZ0.8b.i: State the type of reaction occurring during the titration.
-
18M.3.hl.TZ1.15b:
Dye-sensitized solar cells, DSSCs, use a dye to absorb the sunlight. State two advantages that DSSCs have over traditional silicon based photovoltaic cells.
-
18M.3.hl.TZ1.4c.i:
Draw the structure of the monomer from which nylon-6 is produced by a condensation reaction.
-
18M.3.hl.TZ2.25:
Taxol was originally obtained from the bark of the Pacific yew tree.
Outline how Green Chemistry has improved the process of obtaining Taxol.
-
18M.3.hl.TZ2.4a.i:
Deduce the number of atoms per unit cell in vanadium.
-
18M.3.hl.TZ2.4b.i:
Vanadium and other transition metals can interfere with cell metabolism.
State and explain one process, other than by creating free radicals, by which transition metals interfere with cell metabolism.
-
18M.3.hl.TZ1.10a:
Outline why anthocyanins are coloured.
-
18M.3.hl.TZ1.10b:
Explain why the blue colour of a quinoidal base changes to the red colour of a flavylium cation as pH decreases.
-
18M.3.hl.TZ1.13a:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
18M.3.hl.TZ1.13b:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
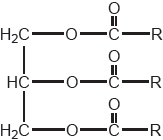
-
18M.3.hl.TZ1.14a.i:
Complete the half-equations on the diagram and identify the species moving between the electrodes.
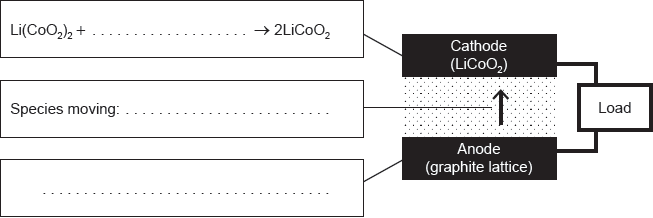
-
18M.3.hl.TZ1.14a.ii:
State the factor that limits the maximum current that can be drawn from this cell and how electrodes are designed to maximize the current.
-
18M.3.hl.TZ1.14b.ii:
Explain how the proportion of 235U in natural uranium is increased.
-
18M.3.hl.TZ1.15a:
Early photovoltaic cells were based on silicon containing traces of other elements. State the type of semiconductor produced by doping silicon with indium, In, giving a reason that refers to its electronic structure.
-
18M.3.hl.TZ1.15c:
The structure of two dyes used in DSSCs are shown.
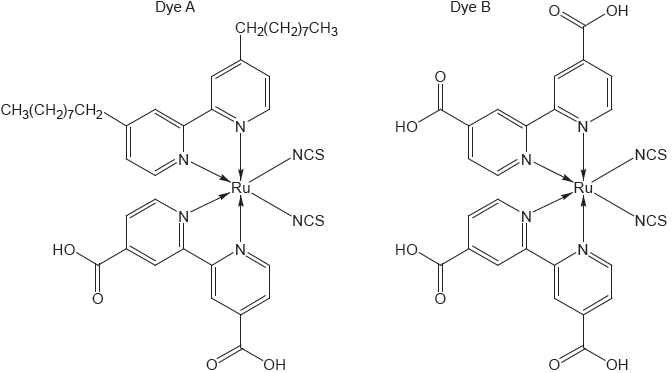
Predict, giving a reason, which dye will absorb light of longer wavelength.
-
18M.3.hl.TZ1.16e:
Many drugs are chiral. Explain how a polarimeter can be used to determine the relative proportion of two enantiomers.
-
18M.3.hl.TZ1.19a:
Describe how ionizing radiation destroys cancer cells.
-
18M.3.hl.TZ1.19b:
Outline how Targeted Alpha Therapy (TAT) is used for treating cancers that have spread throughout the body.
-
18M.3.hl.TZ1.20a:
Hexane and propanone have vapour pressures of 17 kPa and 24 kPa respectively at 20 °C.
Calculate the vapour pressure, in kPa, at 20 °C of a mixture containing 60% hexane and 40% propanone by mole fraction, using Raoult’s law and assuming the mixture is ideal.
-
18M.3.hl.TZ1.20b:
Explain how hexane and propanone may be separated by fractional distillation.
-
18M.3.hl.TZ1.4c.ii:
Deduce, giving a reason, whether the atom economy of a condensation polymerization, such as this, would be greater or less than an addition polymerization, such as the formation of HDPE.
-
18M.3.hl.TZ1.5b:
The diagram illustrates the crystal structure of aluminium metal with the unit cell indicated. Outline the significance of the unit cell.
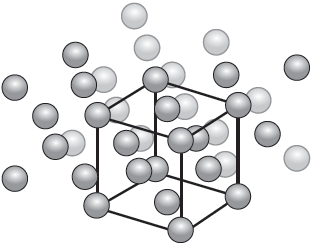
-
18M.3.hl.TZ1.5e:
The concentration of aluminium in drinking water can be reduced by precipitating aluminium hydroxide. Calculate the maximum concentration of aluminium ions in water of pH 7 at 298 K. Solubility product of aluminium hydroxide = 3.3 × 10−34 at 298 K.
-
18M.3.hl.TZ2.11a:
Hemoglobin’s oxygen dissociation curve is shown at a given temperature. Sketch the curve on the graph at a higher temperature.
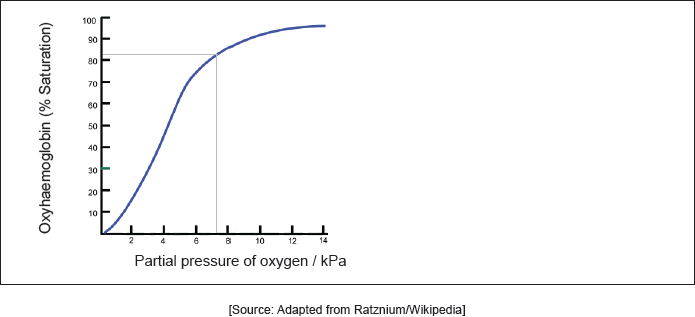
-
18M.3.hl.TZ2.13c:
Fuel cells have a higher thermodynamic efficiency than octane. The following table gives some information on a direct methanol fuel cell.

Determine the thermodynamic efficiency of a methanol fuel cell operating at 0.576 V.
Use sections 1 and 2 of the data booklet.
-
18M.3.hl.TZ2.26b:
The half-life of phosphorus-32 is 14.3 days. Calculate the mass, in g, of 32P remaining after 57.2 days if the initial sample contains 2.63 × 10−8 mol. Use table 1 of the data booklet and Mr = 31.97 g mol−1.
-
18M.3.hl.TZ1.5c:
When X-rays of wavelength 0.154 nm are directed at a crystal of aluminium, the first order diffraction pattern is observed at 18°. Determine the separation of layers of aluminium atoms in the crystal, in m, using section 1 of the data booklet.
-
18M.3.hl.TZ1.6d:
Describe how DNA determines the primary structure of a protein such as insulin.
-
18M.3.hl.TZ1.8b:
Outline why cellulose fibres are strong.
-
18M.3.hl.TZ2.11b:
Outline two differences between normal hemoglobin and foetal hemoglobin.
-
18M.3.hl.TZ2.18a:
Draw the Lewis (electron dot) structure for an appropriate doping element in the box in the centre identifying the type of semiconductor formed.
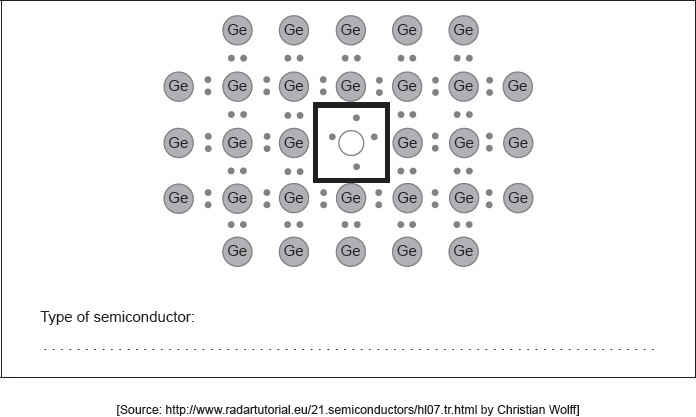
-
18M.3.hl.TZ2.18b.ii:
Outline why complex B absorbs light of longer wavelength than complex A.
-
18M.3.hl.TZ2.26c:
Explain the targeted alpha therapy (TAT) technique and why it is useful.
-
18M.3.hl.TZ2.5c.i:
Distinguish between the manufacture of polyester and polyethene.
-
18M.3.hl.TZ1.5d.i:
Deduce what the shape of the graph indicates about aluminium.
-
18M.3.hl.TZ1.9b:
Outline the significance of the value of the Michaelis constant, Km.
-
18M.3.hl.TZ2.12:
DNA is a biopolymer made up of nucleotides. List two components of a nucleotide.
-
18M.3.hl.TZ2.4a.iii:
Calculate the average mass, in g, of a vanadium atom by using sections 2 and 6 of the data booklet.
-
18M.3.hl.TZ2.6b:
MWCNT are very small in size and can greatly increase switching speeds in a liquid crystal allowing the liquid crystal to change orientation quickly.
Discuss two other properties a substance should have to be suitable for use in liquid crystal displays.
-
18M.3.hl.TZ2.8d:
Calculate the pH of a buffer system with a concentration of 1.25 × 10−3 mol dm−3 carbonic acid and 2.50 × 10−2 mol dm−3 sodium hydrogen carbonate. Use section 1 of the data booklet.
pKa (carbonic acid) = 6.36
-
18M.3.hl.TZ1.9a:
Explain with reference to the binding site on the enzyme how a non-competitive inhibitor lowers the value of Vmax.
-
18M.3.hl.TZ2.10b:
Explain how the structure of vitamin A is important to vision using section 35 of the data booklet.
-
18M.3.hl.TZ2.16c.i:
Calculate the relative rate of effusion of 235UF6(g) to 238UF6(g) using sections 1 and 6 of the data booklet.
-
18M.3.hl.TZ2.18b.i:
State the feature of the molecules responsible for the absorption of light.
-
18M.3.hl.TZ2.26a:
Phosphorous-32 undergoes beta decay. Formulate a balanced nuclear equation for this process.
-
18M.3.hl.TZ2.27a:
Fuel cells use an electrochemical process to determine the concentration of ethanol.
Formulate the overall equation for this process.
-
18M.3.hl.TZ2.4a.ii:
Calculate the expected first order diffraction pattern angle, in degrees, if x-rays of wavelength 150 pm are directed at a crystal of vanadium. Assume the edge length of the crystal to be the same as separation of layers of vanadium atoms found by x-ray diffraction. Use section 1 of the data booklet.
-
18M.3.hl.TZ2.4a.iv:
Determine the volume, in cm3, of a vanadium unit cell.
-
18M.3.hl.TZ1.5d.ii:
Outline why the resistance of aluminium increases above 1.2 K.
-
18M.3.hl.TZ2.16c.ii:
Explain, based on molecular structure and bonding, why diffusion or centrifuging can be used for enrichment of UF6 but not UO2.
-
18M.3.hl.TZ2.4a.v:
Determine the density, in g cm−3, of vanadium by using your answers to (a)(i), (a)(iii) and (a)(iv).
-
18M.3.hl.TZ2.27b:
Predict the chemical shifts and integration for each signal in the 1H NMR spectrum for ethanol using section 27 of the data booklet.
-
18M.3.hl.TZ2.4b.ii:
Vanadium(IV) ions can create free radicals by a Fenton reaction.
Deduce the equation for the reaction of V4+ with hydrogen peroxide.
-
18M.3.hl.TZ2.8c:
Draw the structures of the main form of glycine in buffer solutions of pH 1.0 and 6.0.
The pKa of glycine is 2.34.
-
18M.3.sl.TZ1.3a:
Discuss, in terms of its structure, why an aluminium saucepan is impermeable to water.
-
18M.3.sl.TZ1.3b.i:
State the name given to a material composed of two distinct solid phases.
-
18M.3.sl.TZ1.3b.ii:
State one physical property of HDPE that will be affected by the incorporation of carbon nanotubes.
-
18M.3.sl.TZ1.3b.iii:
Describe how carbon nanotubes are produced by chemical vapour deposition (CVD).
-
18M.3.sl.TZ1.3b.iv:
State the property of carbon nanotubes that enables them to form a nematic liquid crystal phase.
-
18M.3.sl.TZ1.4a:
Both of these are thermoplastic polymers. Outline what this term means.
-
18M.3.sl.TZ1.4b.i:
Compare and contrast the structures of HDPE and LDPE.
-
18M.3.sl.TZ1.4b.ii:
State one way in which a physical property of HDPE, other than density, differs from that of LDPE as a result of this structural difference.
-
18M.3.sl.TZ1.4c.i:
The production of HDPE involves the use of homogeneous catalysts. Outline how homogeneous catalysts reduce the activation energy of reactions.
-
18M.3.sl.TZ1.4c.ii:
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
-
18M.3.sl.TZ1.4d:
Suggest two of the major obstacles, other than collection and economic factors, which have to be overcome in plastic recycling.
-
18M.3.sl.TZ1.4e:
Suggest why there are so many different ways in which plastics can be classified. HDPE can, for example, be categorized thermoplastic, an addition polymer, having Resin Identification Code (RIC) 2, etc.
-
18M.3.sl.TZ1.5:
Aluminium is produced by the electrolysis of a molten electrolyte containing bauxite.
Determine the mass, in g, of aluminium produced by the passage of a charge of 1.296 × 1013 C. Use sections 2 and 6 of the data booklet.
-
18M.3.sl.TZ1.7a.ii:
State one factor that increases the rate at which saturated lipids become rancid.
-
18M.3.sl.TZ1.7c.iv:
Plastics are another source of marine pollution. Outline one way in which plastics can be made more biodegradable.
-
18M.3.sl.TZ1.8b:
A person with diabetes suffering very low blood sugar (hypoglycaemia) may be advised to consume glucose immediately and then eat a small amount of starchy food such as a sandwich. Explain this advice in terms of the properties of glucose and starch.
-
18M.3.sl.TZ1.13d.i:
Morphine and codeine are strong analgesics. Outline how strong analgesics function.
-
18M.3.sl.TZ1.14b:
Explain how omeprazole (Prilosec) reduces stomach acidity.
-
18M.3.sl.TZ1.15b:
Shikimic acid, the precursor for oseltamivir (Tamiflu), was originally extracted from star anise, and is now produced using genetically modified E. coli bacteria.
Suggest one difficulty associated with synthesizing oseltamivir (Tamiflu) from star anise.
-
18M.3.sl.TZ1.7c.i:
Fish oil is an excellent dietary source of omega-3 fatty acids. Outline one impact on health of consuming omega-3 fatty acids.
-
18M.3.sl.TZ1.9a:
Identify one naturally occurring greenhouse gas, other than carbon dioxide or water vapour, and its natural source.
-
18M.3.sl.TZ1.11a.ii:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
-
18M.3.sl.TZ1.11b:
Outline why biofuels are considered more environmentally friendly, even though they produce more carbon dioxide per kJ of energy than petroleum based fuels.
-
18M.3.sl.TZ1.13c.i:
Compare and contrast the IR spectrum of aspirin with that of salicylic acid, using section 26 of the data booklet.
-
18M.3.sl.TZ1.14a.i:
An antacid tablet contains 680 mg of calcium carbonate, CaCO3, and 80 mg of magnesium carbonate, MgCO3.
State the equation for the reaction of magnesium carbonate with hydrochloric acid.
-
18M.3.sl.TZ1.6c.i:
State the name of the process used to break down the insulin protein into its constituent amino acids.
-
18M.3.sl.TZ1.7b:
Butter contains varying proportions of oleic, myristic, palmitic and stearic acids. Explain in terms of their structures why stearic acid has a higher melting point than oleic acid, using section 34 of the data booklet.
-
18M.3.sl.TZ1.7c.iii:
Explain why sharks and swordfish sometimes contain high concentrations of mercury and polychlorinated biphenyls (PCBs).
-
18M.3.sl.TZ1.8a:
State the specific type of linkage formed between α-glucose fragments in both maltose and amylose.
-
18M.3.sl.TZ1.10c.i:
Determine the specific energy and energy density of petrol (gasoline), using data from sections 1 and 13 of the data booklet. Assume petrol is pure octane, C8H18. Octane: molar mass = 114.26 g mol−1, density = 0.703 g cm−3.
-
18M.3.sl.TZ1.6a:
Draw the structural formula of a dipeptide containing the residues of valine, Val, and asparagine, Asn, using section 33 of the data booklet.
-
18M.3.sl.TZ1.6b:
Deduce the strongest intermolecular forces that would occur between the following amino acid residues in a protein chain.
-
18M.3.sl.TZ1.6c.ii:
Outline how the amino acids may be identified from a paper chromatogram.
-
18M.3.sl.TZ1.7a.i:
Identify the type of rancidity occurring in saturated lipids and the structural feature that causes it.
-
18M.3.sl.TZ1.9c:
The concentrations of oxygen and nitrogen in the atmosphere are much greater than those of greenhouse gases. Outline why these gases do not absorb infrared radiation.
-
18M.3.sl.TZ1.10a:
Identify an element, other than carbon and hydrogen, found at significant concentrations in fossil fuels.
-
18M.3.sl.TZ1.10b:
Petroleum contains many hydrocarbons. Explain how these are separated by fractional distillation.
-
18M.3.sl.TZ1.10c.ii:
Outline why the energy available from an engine will be less than these theoretical values.
-
18M.3.sl.TZ1.11a.i:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
18M.3.sl.TZ1.12b:
Suggest one reason why there is opposition to the increased use of nuclear fission reactors.
-
18M.3.sl.TZ1.13a:
Aspirin is often taken to reduce pain, swelling or fever. State one other use of aspirin.
-
18M.3.sl.TZ1.13b.i:
State what is meant by the bioavailability of a drug.
-
18M.3.sl.TZ1.7c.ii:
Predict the solubility of retinol (vitamin A) in body fat, giving a reason. Use section 35 of the data booklet.
-
18M.3.sl.TZ1.9b:
Formulate an equation that shows how aqueous carbon dioxide produces hydrogen ions, H+(aq).
-
18M.3.sl.TZ1.13b.ii:
Outline how the bioavailability of aspirin may be increased.
-
18M.3.sl.TZ1.13c.ii:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ1.13c.iii:
Outline two consequences of prescribing antibiotics such as penicillin unnecessarily.
-
18M.3.sl.TZ1.13c.iv:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ1.13d.ii:
Suggest one reason why codeine is more widely used than morphine as an analgesic.
-
18M.3.sl.TZ1.14a.ii:
Determine the amount, in mol, of hydrochloric acid neutralized by one antacid tablet.
-
18M.3.sl.TZ1.15a:
Oseltamivir (Tamiflu) and zanamivir (Relenza) are used against flu viruses. Explain how these drugs function.
-
18M.3.sl.TZ2.3a:
ICP-OES/MS can be used to analyse alloys and composites. Distinguish between alloys and composites.
-
18M.3.sl.TZ2.3b:
ICP-MS is a reference mode for analysis. The following correlation graphs between ICP-OES and ICP-MS were produced for yttrium and nickel.
Each y-axis shows concentrations calculated by ICP-OES; each x-axis shows concentrations for the same sample as found by ICP-MS.
The line in each graph is y = x.
Discuss the effectiveness of ICP-OES for yttrium and nickel.
-
18M.3.sl.TZ2.3c.i:
Identify the purpose of each graph.
-
18M.3.sl.TZ2.3c.ii:
Calculate, to four significant figures, the concentration, in μg kg−1, of vanadium in oil giving a signal intensity of 14 950.
-
18M.3.sl.TZ2.3c.iii:
Vanadium(V) oxide is used as the catalyst in the conversion of sulfur dioxide to sulfur trioxide.
SO2(g) + V2O5(s) → SO3(g) + 2VO2(s)
O2(g) + 2VO2(s) → V2O5(s)
Outline how vanadium(V) oxide acts as a catalyst.
-
18M.3.sl.TZ2.4a:
Sketch four repeating units of the polymer to show atactic and isotactic polypropene.
-
18M.3.sl.TZ2.4b.i:
State the chemical reason why plastics do not degrade easily.
-
18M.3.sl.TZ2.5a:
State the source of carbon for MWCNT produced by arc discharge and by CVD.
-
18M.3.sl.TZ2.4b.ii:
Compare two ways in which recycling differs from reusing plastics.
-
18M.3.sl.TZ2.5b:
Discuss three properties a substance should have to be suitable for use in liquid crystal displays.
-
18M.3.sl.TZ2.6a:
Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
-
18M.3.sl.TZ2.6c:
Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
-
18M.3.sl.TZ2.6d:
Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
-
18M.3.sl.TZ2.8:
Green Chemistry reduces the production of hazardous materials and chemical waste.
Outline two specific examples or technological processes of how Green Chemistry has accomplished this environmental impact.
-
18M.3.sl.TZ2.9:
Explain the solubility of vitamins A and C using section 35 of the data booklet.
-
18M.3.sl.TZ2.11a:
Explain the molecular mechanism by which carbon dioxide acts as a greenhouse gas.
-
18M.3.sl.TZ2.12b:
Power plants generating electricity by burning coal to boil water operate at approximately 35% efficiency.
State what this means and suggest why it is lower than the thermal efficiency.
-
18M.3.sl.TZ2.14a:
Deduce the equation for the transesterification reaction of pentyl octanoate, C7H15COOC5H11, with methanol.
-
18M.3.sl.TZ2.15:
Drug testing is necessary to determine safe and effective doses.
Distinguish between the lethal dose (LD50) and the toxic dose (TD50).
-
18M.3.sl.TZ2.16b:
State the type of reaction used to synthesize aspirin from salicylic acid.
-
18M.3.sl.TZ2.16c:
Explain why aspirin is not stored in a hot, humid location.
-
18M.3.sl.TZ2.17:
Morphine and diamorphine (heroin) are both opioids.
Explain why diamorphine is more potent than morphine using section 37 of the data booklet.
-
18M.3.sl.TZ2.20:
Drug synthesis often involves solvents.
Identify a common hazardous solvent and a Green solvent that could replace it.
-
18M.3.sl.TZ2.4c:
Civilizations are often characterized by the materials they use.
Suggest an advantage polymers have over materials from the iron age.
-
18M.3.sl.TZ2.6b:
Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon–carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid Mr = 304.5)
-
18M.3.sl.TZ2.6f:
Explain why lipids provide more energy than carbohydrates and proteins.
-
18M.3.sl.TZ2.10c.i:
Outline how higher octane fuels help eliminate “knocking” in engines.
-
18M.3.sl.TZ2.6e:
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
-
18M.3.sl.TZ2.7a:
Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
-
18M.3.sl.TZ2.7c:
Outline why amino acids have high melting points.
-
18M.3.sl.TZ2.10a:
Outline two reasons why oil is one of the world’s significant energy sources.
-
18M.3.sl.TZ2.10b.i:
Formulate an equation for the cracking of C16H34 into two products with eight carbon atoms each.
-
18M.3.sl.TZ2.10b.ii:
Identify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
-
18M.3.sl.TZ2.10c.ii:
The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
-
18M.3.sl.TZ2.11b:
Discuss the significance of two greenhouse gases, other than carbon dioxide, in causing global warming or climate change.
-
18M.3.sl.TZ2.12a:
Calculate the thermal efficiency of a steam turbine supplied with steam at 540°C and using a river as the choice of sink at 23 °C.
-
18M.3.sl.TZ2.13a:
Compare and contrast the process of nuclear fusion with nuclear fission.
-
18M.3.sl.TZ2.13b:
Dubnium-261 has a half-life of 27 seconds and rutherfordium-261 has a half-life of 81 seconds.
Estimate what fraction of the dubnium-261 isotope remains in the same amount of time that of rutherfordium-261 decays.
-
18M.3.sl.TZ2.14b:
Outline why the ester product of this reaction is a better diesel fuel than pentyl octanoate.
-
18M.3.sl.TZ2.16a.i:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ2.16a.ii:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ2.18a:
Formulate a chemical equation for the neutralization of stomach acid with calcium carbonate.
-
18M.3.sl.TZ2.18b:
Calculate the amount, in mol, of stomach acid neutralized by an antacid tablet containing 0.750 g calcium carbonate.
-
18M.3.sl.TZ2.18c:
Explain how omeprazole (Prilosec) regulates pH in the stomach.
-
18M.3.sl.TZ2.19a:
Identify the names of two functional groups present in zanamivir using section 37 of the data booklet.
-
18M.3.sl.TZ2.19b:
Distinguish between bacteria and viruses.
-
18M.3.hl.TZ2.8e:
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
-
18M.3.hl.TZ2.8f:
UV-Vis spectroscopy can be used to determine the unknown concentration of a substance in a solution.
Calculate the concentration of an unknown sample of pepsin with an absorbance of 0.725 using section 1 of the data booklet.
Cell length = 1.00 cm
Molar absorptivity (extinction coefficient) of the sample = 49650 dm3 cm−1 mol−1
-
18M.3.hl.TZ2.8g:
A different series of pepsin samples is used to develop a calibration curve.
Estimate the concentration of an unknown sample of pepsin with an absorbance of 0.30 from the graph.
-
18M.3.sl.TZ1.12a.ii:
Explain how 235U fission results in a chain reaction, including the concept of critical mass.
-
18N.3.sl.TZ0.15a:
State one way in which viruses differ from bacteria.
- 18N.3.sl.TZ0.6a: Describe the interaction responsible for the secondary structure of a protein.
-
18N.3.sl.TZ0.10a:
Formulate equation(s) for the conversion of coal and steam to methane.
- 18N.3.sl.TZ0.13c: Outline the meaning of the bioavailability of a drug.
-
18N.3.hl.TZ0.2b:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
- 18N.3.hl.TZ0.10c.i: Outline the difference between their structures.
-
18N.3.sl.TZ0.14a:
Determine the pH of a buffer solution that is 0.0100 mol dm−3 sodium hydrogen carbonate and 0.0200 mol dm−3 sodium carbonate, using section 1 of the data booklet.
Ka (hydrogen carbonate ion) = 4.8 × 10−11
-
18N.3.sl.TZ0.16:
Suggest two reasons why chlorinated solvents should neither be released into the atmosphere nor incinerated (burnt).
- 18N.3.sl.TZ0.5a: The formation of proteins from amino acids is an example of an anabolic reaction in the human...
-
18N.3.sl.TZ0.2b.ii:
In an incomplete combustion of the polyvinyl chloride, PVC, it was found that hydrogen chloride, carbon monoxide, carbon dioxide, and water vapour were released.
Formulate an equation for this reaction using the formula of the PVC repeating unit.
-
18N.3.hl.TZ0.13a:
Formulate equation(s) for the conversion of coal and steam to methane.
-
18N.3.hl.TZ0.15d.iv:
Deduce the reduction half-equation at the cathode.
-
18N.3.sl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
- 18N.3.sl.TZ0.7c: Outline one effect of increased levels of low-density lipoproteins in the blood.
-
18N.3.sl.TZ0.9c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
- 18N.3.hl.TZ0.10b: Outline how the two monomer structures, galactose and glucose, differ.
-
18N.3.hl.TZ0.13c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
- 18N.3.sl.TZ0.12c: Outline one effect of over-prescription of penicillin.
- 18N.3.sl.TZ0.11a: Suggest another advantage and one disadvantage of solar energy.
- 18N.3.hl.TZ0.15d.ii: Outline the effect of sunlight on the dye in the solar cell.
- 18N.3.hl.TZ0.17b: Outline the meaning of the bioavailability of a drug.
-
18N.3.sl.TZ0.3b:
An unknown antacid sample has a lead ion concentration of 0.50 μg dm‒3.
Calculate the concentration of lead ions in the sample in mol dm‒3.
- 18N.3.sl.TZ0.9b.i: Outline why the term breeder is used for the reactors.
- 18N.3.hl.TZ0.16b: Explain how the open β-lactam ring kills bacteria.
- 18N.3.hl.TZ0.7a: State the feature of DNA that determines the primary structure of proteins synthesised by a cell.
- 18N.3.sl.TZ0.2c.i: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
-
18N.3.hl.TZ0.11b:
Explain why carbon monoxide is very toxic and how it may be possible to treat carbon monoxide poisoning.
-
18N.3.sl.TZ0.11e:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
-
18N.3.sl.TZ0.14b:
State the equation for the reaction of calcium carbonate, the active ingredient in some antacids, with stomach acid.
-
18N.3.hl.TZ0.22b.ii:
Suggest why the percentage of technetium-99m remaining in the human body two days after injection will be lower than that calculated in (b)(i).
-
18N.3.hl.TZ0.3d.ii:
An aqueous lead(II) ion reacts with three ethane-1,2-diamine molecules to form an octahedral chelate ion.
Outline why the chelate ion is more stable than the reactants.
-
18N.3.sl.TZ0.10b.ii:
Comment on the specific energies of hydrogen and methane.
-
18N.3.sl.TZ0.10b.i:
Calculate the specific energy, in kJ g−1, of methane.
- 18N.3.sl.TZ0.12a: State the internal bond angles in the β-lactam ring and the expected bond angles for the same...
- 18N.3.sl.TZ0.12e: Suggest why human cells are not affected by penicillin.
-
18N.3.hl.TZ0.14d:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
- 18N.3.hl.TZ0.8b: Explain the action of an enzyme and state one of its limitations.
- 18N.3.sl.TZ0.11c.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
- 18N.3.sl.TZ0.12b: Explain how the open β-lactam ring kills bacteria.
- 18N.3.sl.TZ0.12d: State how the structure of penicillin can be changed to combat this effect.
- 18N.3.sl.TZ0.14c: Suggest a technique for measuring the percentage mass of calcium carbonate in this type of...
-
18N.3.hl.TZ0.21a:
The diagram shows part of a Taxol molecule in skeletal form.
Draw a circle around each chiral carbon.
- 18N.3.sl.TZ0.6b.ii: Enzymes are widely used in washing detergents. Outline how they improve the efficiency of the...
-
18N.3.sl.TZ0.7b.i:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
- 18N.3.hl.TZ0.11a: A graph showing saturation of oxygen against partial pressure of oxygen is shown. Explain the...
- 18N.3.sl.TZ0.11d: Contrast the importance of carbon dioxide and methane as greenhouse gases.
-
18N.3.sl.TZ0.13a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
-
18N.3.sl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
-
18N.3.sl.TZ0.4c:
Arc discharge, consisting of two inert metal electrodes in a liquid solvent, is one method of producing carbon nanotubes (CNTs).
Predict, giving a reason, the electrode at which the solvent cyclohexane, C6H12, will decompose to form CNTs.
- 18N.3.sl.TZ0.8a: Name the type of link between the two monosaccharide residues.
- 18N.3.hl.TZ0.12d.ii: Explain why free radicals are harmful to living cells.
-
18N.3.hl.TZ0.13b:
Comment on the specific energies of hydrogen and methane.
-
18N.3.hl.TZ0.15c:
A voltaic cell consists of a nickel electrode in 1.0 mol dm−3 Ni2+ (aq) solution and a cadmium electrode in a Cd2+ (aq) solution of unknown concentration.
Cd (s) + Ni2+ (aq) → Cd2+ (aq) + Ni (s) EΘcell = 0.14 V
Determine the concentration of the Cd2+ (aq) solution if the cell voltage, E, is 0.19 V at 298 K. Use section 1 of the data booklet.
- 18N.3.hl.TZ0.15d.i: Identify the structural feature of the dye that allows the conversion of solar energy into...
-
18N.3.hl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
- 18N.3.hl.TZ0.2d.i: State the names of the two terminal functional groups in X.
- 18N.3.sl.TZ0.4a: Outline two observations that he could have made.
- 18N.3.sl.TZ0.7b.ii: State two functions of lipids in the body.
-
18N.3.hl.TZ0.12d.i:
Deduce a Lewis (electron dot) structure of the superoxide, O2–, free radical.
-
18N.3.hl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
- 18N.3.sl.TZ0.11b: Light can be absorbed by chlorophyll and other pigments. Consider molecules A and B represented...
- 18N.3.sl.TZ0.15b: Outline two different ways in which antiviral medications work.
- 18N.3.sl.TZ0.7a: A phospholipid generally consists of two hydrophobic fatty acids and a hydrophilic...
- 18N.3.hl.TZ0.14c: Contrast the importance of carbon dioxide and methane as greenhouse gases.
- 18N.3.sl.TZ0.11c.ii: Describe how vegetable oils can be converted to a more suitable fuel.
-
18N.3.sl.TZ0.2d:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
- 18N.3.sl.TZ0.8b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.hl.TZ0.10a: Name the type of link between the two monosaccharide residues.
- 18N.3.hl.TZ0.9c: Outline one effect of increased levels of low-density lipoproteins in the blood.
- 18N.3.sl.TZ0.13b: Describe the analgesic action of an opiate.
- 18N.3.sl.TZ0.5c: Explain how a xenobiotic is biomagnified.
- 18N.3.sl.TZ0.9a: Explain fusion reactions with reference to binding energy.
- 18N.3.hl.TZ0.2c: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
- 18N.3.sl.TZ0.3a: State the type of particle present in the plasma formed.
- 18N.3.hl.TZ0.10c.ii: Outline why cellulose is an essential part of human diet.
- 18N.3.hl.TZ0.7b: Suggest one concern about the use of genetically modified, GM, food.
-
18N.3.sl.TZ0.10c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
-
18N.3.sl.TZ0.2b.i:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
- 18N.3.hl.TZ0.12a: Explain fusion reactions with reference to binding energy.
-
18N.3.hl.TZ0.12c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
- 18N.3.hl.TZ0.16c: State how the structure of penicillin can be modified to combat the effect of resistance caused...
-
18N.3.hl.TZ0.23a:
State an analytical technique used to separate anabolic steroids from other compounds in an athlete’s urine or blood.
-
18N.3.hl.TZ0.23b:
Ethanol in breath can be detected by a redox reaction. Outline this method of detection. An equation is not required.
- 18N.3.sl.TZ0.2c.ii: Identify another advantage of using a zeolite instead of concentrated sulfuric acid.
-
18N.3.sl.TZ0.5b:
Suggest why it is advisable for those living in northerly or southerly latitudes (that is away from the equator) to take vitamin D supplements during the winter.
- 18N.3.hl.TZ0.14b.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
-
18N.3.hl.TZ0.17a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
- 18N.3.hl.TZ0.2d.ii: Deduce the repeating unit of the polymer of X.
-
18N.3.hl.TZ0.22a:
Alpha particles are more damaging to human cells than any other nuclear radiation and yet they are used in targeted alpha therapy (TAT).
Explain how TAT is relatively safe to use in the treatment of dispersed cancers.
-
18N.3.hl.TZ0.3b.i:
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
18N.3.hl.TZ0.3b.ii:
Lead ions are toxic and can be precipitated using hydroxide ions.
Pb2+ (aq) + 2OH‒ (aq) Pb(OH)2 (s)
Sufficient sodium hydroxide solid is added to the antacid sample to produce a 1.0 × 10‒2 mol dm‒3 hydroxide ion solution at 298 K.
Deduce if a precipitate will be formed, using section 32 of the data booklet.
If you did not calculate the concentration of lead ions in (b)(i), use the value of 2.4 × 10−4 mol dm‒3, but this is not the correct value.
- 18N.3.sl.TZ0.4b: The structure of biphenyl nitrile is shown. Describe, giving a reason, a feature of the...
- 18N.3.hl.TZ0.15d.iii: State the purpose of TiO2.
- 18N.3.hl.TZ0.3a: State the type of particle present in the plasma formed.
- 18N.3.sl.TZ0.6b.i: Explain the action of an enzyme and state one of its limitations.
- 18N.3.hl.TZ0.12b.i: Outline why the term breeder is used for the reactors.
- 18N.3.hl.TZ0.16a: State the internal bond angles in the b-lactam ring and the expected bond angles in sp2 and sp3...
-
18N.3.hl.TZ0.5a.ii:
Calculate the number of atoms per unit cell of gold, showing your working.
-
18N.3.hl.TZ0.15b:
Formulate half-equations for the reactions at the anode (negative electrode) and cathode (positive electrode) during discharge of a lithium-ion battery.
- 18N.3.hl.TZ0.16d: Suggest why human cells are not affected by penicillin.
- 18N.3.hl.TZ0.19: Outline two different ways in which antiviral medications work.
- 18N.3.sl.TZ0.9b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
- 18N.3.hl.TZ0.12b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
- 18N.3.hl.TZ0.8a: Describe the interaction responsible for the secondary structure of a protein.
- 18N.3.hl.TZ0.14a: Suggest another advantage and one disadvantage of solar energy.
- 18N.3.hl.TZ0.14b.ii: Describe how vegetable oils can be converted to a more suitable fuel.
- 18N.3.hl.TZ0.15a: Outline how a rechargeable battery differs from a primary cell.
- 18N.3.hl.TZ0.21b: Outline how chiral auxiliaries are used to synthesize the desired enantiomer.
- 18N.3.hl.TZ0.21c: Explain the process of solvent extraction by which Taxol is isolated.
-
18N.3.hl.TZ0.22b.i:
Technetium-99m () has a half-life of 6.0 hours. Calculate the percentage of remaining in a sample of the radioisotope after two days.
- 18N.3.hl.TZ0.3d.i: State one feature of a chelating agent.
- 18N.3.hl.TZ0.5a.i: State the name of the crystal structure of gold.
-
18N.3.hl.TZ0.5b:
The edge length of the gold unit cell is 4.08 × 10‒8 cm.
Determine the density of gold in g cm‒3, using sections 2 and 6 of the data booklet.
-
18N.3.hl.TZ0.8c:
Contrast the actions of non-competitive and competitive inhibitors of an enzyme and state their effects on the maximum rate of reaction, Vmax, and the Michaelis–Menten constant, Km.
-
18N.3.hl.TZ0.9a:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
- 18N.3.hl.TZ0.9b: State two functions of lipids in the body.
-
17M.3.hl.TZ1.25c:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
17M.3.hl.TZ1.25a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.hl.TZ1.25e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
17M.3.hl.TZ1.25b:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
18N.3.hl.TZ0.2d.iii:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
-
19M.3.hl.TZ1.4e:
State one factor considered when making green chemistry polymers.
-
19M.3.hl.TZ1.3e:
Lithium forms a crystalline lattice with the unit cell structure shown below.
X-ray diffraction shows that the length of the edge of the unit cell is 3.51 × 10−8 cm.
Determine the density of lithium, in g cm−3, using sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.hl.TZ1.4c:
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
-
19M.3.hl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.hl.TZ1.4d:
Classify polybutadiene as either an addition or condensation polymer, giving a reason.
-
19M.3.hl.TZ1.3b(iii):
Suggest a better method.
-
19M.3.hl.TZ1.9a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.hl.TZ1.9b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.hl.TZ1.12a(i):
A Michaelis–Menten plot for an enzyme-catalysed reaction is shown.
Sketch a curve to show the effect of a competitive inhibitor.
-
19M.3.hl.TZ1.16a(ii):
Outline why the reaction releases energy.
-
19M.3.hl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.hl.TZ1.8d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.hl.TZ1.9c:
Aspartic acid is obtained synthetically as a racemic mixture. Draw the three‑dimensional shape of each isomer showing their spatial relationship to each other. Use section 33 of the data booklet.
-
19M.3.hl.TZ1.3a:
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.hl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.hl.TZ1.15c(i):
Methane can also be obtained by fractional distillation of crude oil.
[Source: Image used with kind permission of science-resources.co.uk]
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.hl.TZ1.16a(iii):
The masses of the particles involved in this fission reaction are shown below.
Mass of neutron = 1.00867 amu
Mass of U-235 nucleus = 234.99346 amu
Mass of Ba-144 nucleus = 143.89223 amu
Mass of Kr-89 nucleus = 88.89788 amuDetermine the energy released, in J, when one uranium-235 nucleus undergoes fission. Use this data and information from sections 1 and 2 of the data booklet.
-
19M.3.hl.TZ1.21a:
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.hl.TZ1.23b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.hl.TZ1.24a(ii):
Technetium-99 decays further, emitting beta radiation. Formulate the equation for the decay of technetium-99.
-
19M.3.hl.TZ1.24b(i):
Outline what is meant by low-level waste.
-
19M.3.hl.TZ1.3d(i):
Lithium has shown some superconductive properties when doped into graphene or when under high pressure. Under high pressure, however, the Meissner effect is absent.
Describe the Meissner effect.
-
19M.3.hl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.hl.TZ1.10b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.hl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.hl.TZ1.7b:
State the number of coordinate covalent bonds EDTA forms with Ni2+.
-
19M.3.hl.TZ1.14b:
Outline one benefit of the use of these products.
-
19M.3.hl.TZ1.15d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.hl.TZ1.3b(ii):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.hl.TZ1.3d(ii):
At very low temperatures, lithium atoms enhance the phonon binding of electrons in graphene suggesting the formation of Cooper pairs.
Explain how Cooper pairs are formed.
-
19M.3.hl.TZ1.8c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.hl.TZ1.11a:
The absorption spectrum of β-carotene is shown below.
Explain its colour in terms of its absorption bands. Use section 17 of the data booklet.
-
19M.3.hl.TZ1.5a:
Label with an asterisk, *, the chiral carbon atom.
-
19M.3.hl.TZ1.23a:
Explain how opiates act to provide pain relief.
-
19M.3.hl.TZ1.7a:
Explain how entropy affects this equilibrium.
-
19M.3.hl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19M.3.hl.TZ1.22c:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.hl.TZ1.11b:
The absorption spectrum of chlorophyll a is shown below.
Suggest how the combination of chlorophyll a and carotenoids is beneficial for photosynthesis.
-
19M.3.hl.TZ1.22a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.hl.TZ1.19b(ii):
Identify the splitting pattern of signals X and Y.
X:
Y:
-
19M.3.hl.TZ1.25a:
Identify the chiral carbon atom using an asterisk, *.
-
19M.3.hl.TZ1.10a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.hl.TZ1.14a:
Outline what is meant by genetically modified organisms.
-
19M.3.hl.TZ1.15b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.hl.TZ1.15b(ii):
Hydroelectric power plants produced 16% of the world’s energy in 2015, down from 21% in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.hl.TZ1.17b(i):
Ethanol can be used in a direct-ethanol fuel cell (DEFC) as illustrated by the flow chart.
Deduce the half-equations occurring at electrodes A and B.
Electrode A:
Electrode B:
-
19M.3.hl.TZ1.22b:
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.hl.TZ1.25b:
Enantiomers can be identified using a polarimeter. Outline how this instrument differentiates the enantiomers.
-
19M.3.hl.TZ1.8b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.hl.TZ1.10c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline one effect of trans-fatty acids on health.
-
19M.3.hl.TZ1.15d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.hl.TZ1.17c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.hl.TZ1.18a:
Some solar cells use photovoltaic semi-conductors. Compare, giving reasons, the electrical conductivity of metals and semi-conductors as temperature increases.
-
19M.3.hl.TZ1.19b(i):
Deduce the protons responsible for signals X and Y by marking them on the structure of aspirin in (a). Use section 27 of the data booklet.
-
19M.3.hl.TZ1.24b(ii):
Outline the disposal of LLW.
-
19M.3.hl.TZ1.24c:
Magnetic resonance imaging (MRI) is an application of NMR technology using radiowaves.
Suggest why MRI is much less dangerous than imaging techniques such as X-rays and radiotracers. Use section 3 of the data booklet.
-
19M.3.hl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase.
Shape of molecules:
Distribution:
-
19M.3.hl.TZ1.12a(ii):
Suggest, based on the Michaelis–Menten plot, how a competitive inhibitor such as ethanol reduces the toxicity of methanol.
-
19M.3.hl.TZ1.16b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.hl.TZ1.17b(ii):
State the name and function of X in the diagram in (b)(i).
Name:
Function:
-
19M.3.hl.TZ1.19a:
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that diff erentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.hl.TZ1.24a(i):
Determine the percentage of technetium-99m remaining after 24.0 hours.
-
19M.3.hl.TZ1.12b:
Enzymatic activity is studied in buffered aqueous solutions.
Calculate the ratio in which 0.1 mol dm−3 NaH2PO4 (aq) and 0.1 mol dm−3 Na2HPO4 (aq) should be mixed to obtain a buffer with pH = 6.10. Use section 1 of the data booklet.
pKa (NaH2PO4) = 7.20
-
19M.3.hl.TZ1.15a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.hl.TZ1.8a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.hl.TZ1.15c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.hl.TZ1.16c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for its radioactivity to fall to 10% of its initial value, using section 1 of the data booklet.
-
19M.3.hl.TZ1.22a(ii):
The resulting active metabolite of oseltamivir can be detected by mass spectrometry (MS) analysis.
Deduce the mass of the expected carboxylate ion.
Mr oseltamivir = 312
-
19M.3.hl.TZ1.17b(iii):
Outline why aqueous ethanol, rather than pure ethanol, is used in a DEFC.
-
19M.3.hl.TZ2.10a(i):
Outline which pKa value should be used when calculating the pH of the solution, giving your reason.
-
19M.3.hl.TZ2.13a:
Outline why the complex formed between Fe2+ and oxygen is red. Refer to the diagram above and section 17 of the data booklet.
-
19M.3.hl.TZ2.16a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.hl.TZ2.22b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.hl.TZ1.17a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.hl.TZ1.18b:
Suggest one advantage of a dye-sensitized solar cell (DSSC) over a silicon based photovoltaic cell.
-
19M.3.hl.TZ1.20a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.hl.TZ1.20b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.hl.TZ1.20b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.hl.TZ2.9d(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.hl.TZ1.13:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.hl.TZ1.16a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.hl.TZ1.21b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.hl.TZ2.17:
This question is about biofuel.
Evaluate the use of biodiesel in place of diesel from crude oil.
-
19M.3.hl.TZ2.9a(i):
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.hl.TZ2.19a:
Outline how a microbial fuel cell produces an electric current from glucose.
C6H12O6 (aq) + 6O2 (g) → 6CO2 (g) + 6H2O (l)
- 19M.3.hl.TZ2.19c: Outline one difference between a primary and a secondary cell.
-
19M.3.hl.TZ2.20a:
Sketch graphs to show the general effect of increasing temperature on the electrical conductivity of semiconductors and metals on the axes below.
-
19M.3.hl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.hl.TZ2.11c:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
-
19M.3.hl.TZ2.15a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.hl.TZ2.22c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.hl.TZ2.5e:
Outline, giving a reason, how addition and condensation polymerization compare with regard to green chemistry.
-
19M.3.hl.TZ2.5f:
Draw the full structural formula of the organic functional group formed during the polymerization of the two reactants below.
-
19M.3.hl.TZ2.21b:
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
-
19M.3.hl.TZ2.25a:
Examine the synthesis of taxol in terms of green chemistry criteria.
-
19M.3.hl.TZ2.11a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.hl.TZ2.11b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.hl.TZ2.27a:
Describe how a fuel cell breathalyser works.
-
19M.3.hl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.hl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19M.3.hl.TZ2.5d:
Suggest why the addition of plasticizers is controversial.
-
19M.3.hl.TZ2.26a:
Evaluate the suitability of technetium-99m for this use.
-
19M.3.hl.TZ2.26b:
Calculate the percentage of technetium-99m remaining after 10.0 hours. Use section 1 of the data booklet.
-
19M.3.hl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48 250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ2.9a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.hl.TZ2.12a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.hl.TZ2.12c:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19M.3.hl.TZ2.15c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
-
19M.3.hl.TZ2.16d:
Calculate the half-life of an isotope whose mass falls from 5.0 × 10−5 g to 4.0 × 10−5 g in 31.4 s, using section 1 of the data booklet.
-
19M.3.hl.TZ2.19b:
The cell potential for the spontaneous reaction when standard magnesium and silver half-cells are connected is +3.17 V.
Determine the cell potential at 298 K when:
[Mg2+] = 0.0500 mol dm−3
[Ag+] = 0.100 mol dm−3Use sections 1 and 2 of the data booklet.
-
19M.3.hl.TZ2.23b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19M.3.hl.TZ2.24b:
Discuss two difficulties associated with solving the AIDS problem.
-
19M.3.hl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19M.3.hl.TZ2.9c:
State and explain how a competitive inhibitor affects the maximum rate, Vmax, of an enzyme-catalyzed reaction.
-
19M.3.hl.TZ2.10b:
Describe what is meant by the genetic code and how it relates to protein synthesis.
-
19M.3.hl.TZ2.11a(ii):
Identify the type of reaction in (a).
-
19M.3.hl.TZ2.7a:
State what is meant by a superconductor.
-
19M.3.hl.TZ2.22a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.hl.TZ2.22b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.hl.TZ2.6a:
State the number of atoms in the unit cell.
-
19M.3.hl.TZ2.15b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19M.3.hl.TZ2.18b:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.hl.TZ2.10a(ii):
Calculate the pH of the glutamine solution using section 1 of the data booklet.
- 19M.3.hl.TZ2.13b(ii): Sketch another line to show the effect of an increase in body temperature on the oxygen...
-
19M.3.hl.TZ2.23a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.hl.TZ2.23a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
-
19M.3.hl.TZ2.5c:
Explain how plasticizers affect the properties of plastics.
-
19M.3.hl.TZ2.8a:
Outline why heavy metals are toxic.
-
19M.3.hl.TZ2.8b:
Determine the maximum concentration of lead(II) ions at 298 K in a solution in which the concentration of carbonate ions is maintained at 1.10 × 10−4 mol dm−3. Use section 32 of the data booklet.
-
19M.3.hl.TZ2.13b(i):
Explain the shape of the curve.
-
19M.3.hl.TZ2.25b:
Outline the operation of a polarimeter used to distinguish between enantiomers.
-
19M.3.hl.TZ2.6b:
Determine the density of calcium, in g cm−3, using section 2 of the data booklet.
Ar = 40.08; metallic radius (r) = 1.97 × 10−10 m
-
19M.3.hl.TZ2.16a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.hl.TZ2.21a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
-
19M.3.hl.TZ2.8c:
State a method, other than precipitation, of removing heavy metal ions from solution.
-
19M.3.hl.TZ2.11d:
Phospholipids are also found in lipoprotein structures.
Describe one effect of increased levels of low-density lipoprotein (LDL) on health.
-
19M.3.hl.TZ2.9b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19M.3.hl.TZ2.9d(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
-
19M.3.hl.TZ2.14:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.hl.TZ2.16a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.hl.TZ2.20b:
Explain the function of dyes in a dye-sensitized solar cell (DSSC).
-
19M.3.hl.TZ2.12b:
Classify, giving your reason, the hexose (six-membered) ring of sucrose as an α or β isomer.
-
19M.3.hl.TZ2.16b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
-
19M.3.hl.TZ2.16c:
Outline how the energy of a fission reaction can be calculated.
-
19M.3.hl.TZ2.18a:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.hl.TZ2.27b:
Alcohol levels in the breath can also be determined using IR spectroscopy.
Suggest, giving a reason, which bond’s absorbance is most useful for detecting ethanol in breath.
Bond:
Reason:
-
19M.3.hl.TZ2.22d:
State why aspirin should not be taken with alcohol.
-
19M.3.hl.TZ2.24a:
Outline one way in which antiviral drugs work.
-
19M.3.sl.TZ1.11b(ii):
Hydroelectric power plants produced 16 % of the world’s energy in 2015, down from 21 % in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.sl.TZ1.8a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.sl.TZ1.11c(i):
Methane can also be obtained by fractional distillation of crude oil.
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.sl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.sl.TZ1.14:
Aspirin can be obtained from salicylic acid.
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that differentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.sl.TZ1.19b:
Outline the disposal of LLW.
-
19M.3.sl.TZ1.3b(i):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.sl.TZ1.4c(i):
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.sl.TZ1.7b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.sl.TZ1.10:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.sl.TZ1.13c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.sl.TZ1.11a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.sl.TZ1.11d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.sl.TZ1.19a:
Outline what is meant by low-level waste.
-
19M.3.sl.TZ1.3b(ii):
Suggest a better method.
-
19M.3.sl.TZ1.13a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.sl.TZ1.16a(ii):
Determine the volume of CO2 (g), in dm3, produced at STP, when 1.00 g of CaCO3 (s) reacts completely with stomach acid.
Mr CaCO3 = 100.09
-
19M.3.sl.TZ1.9a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.sl.TZ1.18a:
Explain how opiates act to provide pain relief.
-
19M.3.sl.TZ1.18b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.sl.TZ1.12c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for the mass of 89Kr to fall to 6.25 % of its initial value.
-
19M.3.sl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.sl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.sl.TZ1.7c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.sl.TZ1.11b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.sl.TZ1.16b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.sl.TZ1.17a(ii):
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.sl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.sl.TZ1.7d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.sl.TZ1.4c(ii):
The trace quantities of dioxins from tyre fires are rarely inhaled and instead settle on the ground.
Describe why this is a health concern.
-
19M.3.sl.TZ1.7a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.sl.TZ1.11c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.sl.TZ1.12a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.sl.TZ1.15b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.sl.TZ1.17b:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.sl.TZ1.3a(ii):
Explain why lithium is paramagnetic while lithium hydride is diamagnetic by referring to electron configurations.
-
19M.3.sl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.sl.TZ1.5a:
State the name of the functional group which allows the molecule to be responsive to applied electric fields.
-
19M.3.sl.TZ1.11d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.sl.TZ1.16a(i):
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.sl.TZ1.3a(i):
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.sl.TZ1.8b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.sl.TZ1.9c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline two effects of trans-fatty acids on health.
-
19M.3.sl.TZ1.12a(ii):
Outline why the reaction releases energy.
-
19M.3.sl.TZ1.9b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.sl.TZ1.13b:
Show that, for combustion of equal masses of fuel, ethanol (Mr = 46 g mol−1) has a lower carbon footprint than octane (Mr = 114 g mol−1).
-
19M.3.sl.TZ1.15a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.sl.TZ1.17a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.sl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.sl.TZ1.15b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.sl.TZ1.12b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.sl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.sl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19M.3.sl.TZ2.7a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.sl.TZ2.10c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
-
19M.3.sl.TZ2.15d:
State why aspirin should not be taken with alcohol.
-
19M.3.sl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.sl.TZ2.6a(i) :
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.sl.TZ2.6a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.sl.TZ2.7b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.sl.TZ2.8a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.sl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19M.3.sl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.sl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19M.3.sl.TZ2.6c(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.sl.TZ2.10b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19M.3.sl.TZ2.12b:
Evaluate the use of biodiesel in place of diesel from crude oil.
Strength:
Limitation:
-
19M.3.sl.TZ2.13c:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.sl.TZ2.7a(ii):
Identify the type of reaction in (a).
-
19M.3.sl.TZ2.7d:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
-
19M.3.sl.TZ2.14b(i):
State one advantage of using morphine as an analgesic.
-
19M.3.sl.TZ2.15a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.sl.TZ2.5c:
Identify a hazardous product of the incineration of polychloroethene.
-
19M.3.sl.TZ2.13a:
State one greenhouse gas, other than carbon dioxide.
-
19M.3.sl.TZ2.14a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
-
19M.3.sl.TZ2.15c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.sl.TZ2.16b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19M.3.sl.TZ2.5d:
Explain how plasticizers affect the properties of plastics.
-
19M.3.sl.TZ2.5e:
Suggest why the addition of plasticizers is controversial.
-
19M.3.sl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase and the effect that an electric field has on it.
Shape of molecules:Distribution:
Effect of electric field:
-
19M.3.sl.TZ2.8b:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19M.3.sl.TZ2.9:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.sl.TZ2.14b(ii):
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
-
19M.3.sl.TZ2.6c(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
-
19M.3.sl.TZ2.10a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.sl.TZ2.12a:
The structure of chlorophyll is given in section 35 of the data booklet.
State the feature of the chlorophyll molecule that enables it to absorb light in the visible spectrum.
-
19M.3.sl.TZ2.6b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19M.3.sl.TZ2.15b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.sl.TZ2.7e:
Phospholipids are also found in lipoprotein structures.
Describe two effects of increased levels of low-density lipoprotein (LDL) on health.
-
19M.3.sl.TZ2.7c:
Predict, giving a reason, the relative energy density of a carbohydrate and a lipid of similar molar mass.
-
19M.3.sl.TZ2.11a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.sl.TZ2.16a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.sl.TZ2.17a:
Outline one way in which antiviral drugs work.
-
19M.3.sl.TZ2.17b:
Discuss two difficulties associated with solving the AIDS problem.
-
19M.3.sl.TZ2.11c:
90Sr, a common product of fission, has a half-life of 28.8 years.
Determine the number of years for the activity of a sample of 90Sr to fall to one eighth () of its initial value.
-
19M.3.sl.TZ2.13b:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.sl.TZ2.15e:
Outline two factors which must be considered to assess the greenness of any chemical process.
-
19M.3.sl.TZ2.11a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.sl.TZ2.11a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.sl.TZ2.11b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
-
19M.3.sl.TZ2.15b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.sl.TZ2.16a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
- 19N.3.hl.TZ0.5c: Discuss why the recycling of plastics is an energy intensive process.
- 19N.3.sl.TZ0.4c: Polypropene is a thermoplastic. Outline what is meant by thermoplastic.
-
19N.3.hl.TZ0.11b:
Compare the effects of competitive and non-competitive inhibitors.
- 19N.3.sl.TZ0.9c: Explain why stearic acid has a higher melting point than oleic acid.
-
19N.3.sl.TZ0.5a:
Discuss why different methods of reduction are needed to extract metals.
- 19N.3.sl.TZ0.6b: State how liquid crystals are affected by an electric field.
- 19N.3.sl.TZ0.4d: Discuss why the recycling of plastics is an energy intensive process.
- 19N.3.hl.TZ0.4b: State two differences between Type I and Type II superconductors.
- 19N.3.sl.TZ0.6a: Describe the arrangement of soap molecules in the nematic liquid crystal phase.
- 19N.3.hl.TZ0.10b(ii): Suggest why alanine and glycine separate slightly at pH 6.5.
- 19N.3.sl.TZ0.8a: The graph shows the relationship between the temperature and the rate of an enzyme-catalysed...
- 19N.3.hl.TZ0.16b: Outline what is meant by the degradation of energy.
- 19N.3.sl.TZ0.17a: Suggest one reactant used to prepare aspirin from salicylic acid.
-
19N.3.sl.TZ0.4a:
Draw a section of an isotactic polypropene polymer chain containing four repeating units.
- 19N.3.sl.TZ0.3b: Distinguish between heterogeneous and homogeneous catalysts, giving one difference.
- 19N.3.sl.TZ0.4b: Predict, with a reason, whether isotactic or atactic polypropene has the higher melting point.
-
19N.3.hl.TZ0.18a(iii):
Calculate the heat energy released, in J, by the fusion reaction producing one atom of carbon-12. Use section 2 of the data booklet and E = mc2.
-
19N.3.sl.TZ0.13b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
- 19N.3.hl.TZ0.20d(i): Outline the functions of the dye, TiO2 and the electrolyte in the operation of the...
- 19N.3.hl.TZ0.4a(ii): Suggest why the resistance of metals increases with temperature.
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
- 19N.3.hl.TZ0.14a: The graph shows the change in oxygen partial pressure in blood, measured at different pH...
- 19N.3.sl.TZ0.12b(ii): The 1H NMR spectrum of one of the products has four signals. The integration trace shows a ratio...
- 19N.3.sl.TZ0.19b: Suggest a concern about the disposal of solvents from drug manufacturing.
-
19N.3.sl.TZ0.5b(i):
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
-
19N.3.sl.TZ0.7b:
The isoelectric point of amino acids is the intermediate pH at which an amino acid is electrically neutral.
Suggest why Asp and Phe have different isoelectric points.
- 19N.3.hl.TZ0.13b: Explain how the double-helical structure of DNA is stabilized once formed.
-
19N.3.sl.TZ0.3a:
Describe how a heterogeneous catalyst provides an alternative pathway for a reaction.
- 19N.3.hl.TZ0.4a(i): Outline how resistance to electric currents occurs in metals.
-
19N.3.sl.TZ0.7a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
- 19N.3.sl.TZ0.10b: Explain the biomagnification of the pesticide DDT.
-
19N.3.hl.TZ0.20b(ii):
Suggest how PEM fuel cells can be used to produce a larger voltage than that calculated in (b)(i).
-
19N.3.hl.TZ0.5a:
Draw the structure of the monomers of Kevlar® if the by-product of the condensation polymerization is hydrogen chloride.
-
19N.3.sl.TZ0.8b:
Explain why a change in pH affects the tertiary structure of an enzyme in solution.
-
19N.3.sl.TZ0.9a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
- 19N.3.sl.TZ0.9d(i): State one similarity and one difference in composition between phospholipids and...
-
19N.3.sl.TZ0.16d:
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
- 19N.3.hl.TZ0.27a: State two common side effects of radiotherapy.
-
19N.3.sl.TZ0.3c:
Nanotubes are used to support the active material in nanocatalysts.
Explain why oxygen cannot be used for the chemical vapour deposition (CVD) preparation of carbon nanotubes.
-
19N.3.hl.TZ0.7:
X-ray crystallography of a metal crystal produces a diffraction pattern of bright spots.
Using X-rays of wavelength 1.54 × 10−10 m, the first bright spots were produced at an angle θ of 22.3° from the centre.
Calculate the separation between planes of atoms in the lattice, in meters, using section 1 of the data booklet.
-
19N.3.hl.TZ0.8:
1.40 × 10−3 g of NaOH (s) are dissolved in 250.0 cm3 of 1.00 × 10−11 mol dm−3 Pb(OH)2 (aq) solution.
Determine the change in lead ion concentration in the solution, using section 32 of the data booklet.
-
19N.3.sl.TZ0.9d(ii):
Identify a reagent that hydrolyses triglycerides.
- 19N.3.sl.TZ0.8c: State one use of enzymes in reducing environmental problems.
- 19N.3.sl.TZ0.9b: State one impact on health of the increase in LDL cholesterol concentration in blood.
-
19N.3.sl.TZ0.11a:
Discuss the data.
- 19N.3.sl.TZ0.12b(i): Reforming reactions are used to increase the octane number of a hydrocarbon fuel. Suggest the...
- 19N.3.sl.TZ0.15b: Explain why diamorphine has greater potency than morphine.
- 19N.3.hl.TZ0.27b: Explain why technetium-99m is the most common radioisotope used in nuclear medicine.
-
19N.3.sl.TZ0.5b(ii):
Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
- 19N.3.sl.TZ0.10c: Explain why maltose, C12H22O11, is soluble in water.
- 19N.3.hl.TZ0.10b(i): Describe, using another method, how a mixture of four amino acids, alanine, arginine, glutamic...
- 19N.3.sl.TZ0.13a(ii): Explain why fusion is an exothermic process.
-
19N.3.sl.TZ0.16b:
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
-
19N.3.sl.TZ0.17b:
Aspirin, C6H4(OCOCH3)COOH, is only slightly soluble in water.
Outline, including an equation, how aspirin can be made more water-soluble. Use section 37 in the data booklet.
- 19N.3.sl.TZ0.18b: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
- 19N.3.sl.TZ0.18c: The discovery of penicillins contributed to the development of antibiotics. Explain how the...
- 19N.3.hl.TZ0.25b: Outline the impact of antibiotic waste on the environment.
-
19N.3.hl.TZ0.26b:
Describe how the challenge in (a) was resolved by pharmaceutical companies.
- 19N.3.hl.TZ0.11a: Outline the significance of the Michaelis constant Km.
-
19N.3.hl.TZ0.12b:
The chemical change in stored fats causes rancidity characterized by an unpleasant smell or taste.
Compare hydrolytic and oxidative rancidity.
- 19N.3.hl.TZ0.15b: Compare and contrast the structures of starch and cellulose. One similarity: One difference:
- 19N.3.sl.TZ0.15a: State the names of two functional groups present in all three molecules, using section 37 of the...
- 19N.3.hl.TZ0.25c: Suggest a concern about the disposal of solvents from drug manufacturing.
- 19N.3.hl.TZ0.25d: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
- 19N.3.hl.TZ0.5b: State and explain why plasticizers are added to polymers.
-
19N.3.sl.TZ0.14a:
Write the equation for the complete combustion of ethanol.
-
19N.3.hl.TZ0.24c:
Explain how redox chemistry is used to measure the ethanol concentration in a breathalyser.
- 19N.3.hl.TZ0.26a: State the feature of Taxol that is a major challenge in its synthesis. Use section 37 of the data...
-
19N.3.hl.TZ0.13a:
List two components of nucleotides.
- 19N.3.sl.TZ0.10a: State the name of one functional group common to all three vitamins shown in section 35 of the...
- 19N.3.hl.TZ0.15a: Describe the function of chlorophyll in photosynthesis.
-
19N.3.sl.TZ0.14b:
Outline the evidence that relates global warming to increasing concentrations of greenhouse gases in the atmosphere.
- 19N.3.sl.TZ0.19a: Outline the impact of antibiotic waste on the environment.
-
19N.3.hl.TZ0.24b:
Describe a technique for the detection of steroids in blood and urine.
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
- 19N.3.hl.TZ0.20d(ii): Suggest an advantage of the DSSC over silicon-based photovoltaic cells.
-
19N.3.sl.TZ0.11b:
In a natural gas power station, 1.00 tonne of natural gas produces 2.41 × 104 MJ of electricity.
Calculate the percentage efficiency of the power station.
1 tonne = 1000 kg
Specific energy of natural gas used = 55.4 MJ kg−1 - 19N.3.sl.TZ0.12a: Suggest why a high-octane number fuel is preferable.
- 19N.3.hl.TZ0.20c: Suggest an advantage of the PEM fuel cell over the lead-acid battery for use in cars.
- 19N.3.hl.TZ0.21b: Experimental research on both animals and humans contributes to the development...
-
19N.3.sl.TZ0.18a:
State one difference between bacteria and viruses.
-
19N.3.sl.TZ0.14c:
Explain, including a suitable equation, why biofuels are considered to be carbon neutral.
- 19N.3.sl.TZ0.14d: State the type of reaction that occurs when ethanol reacts with vegetable oil to form biodiesel.
-
19N.3.hl.TZ0.20b(i):
Calculate the cell potential, Eθ, in V, using section 24 of the data booklet.
-
19N.3.hl.TZ0.27c:
25.0 μg of iodine-131, with a half-life of 8.00 days, was left to decay.
Calculate the mass of iodine-131, in μg, remaining after 32.0 days. Use section 1 of the data booklet.
-
19N.3.hl.TZ0.24a:
Infrared (IR) spectroscopy is used to identify functional groups in organic compounds.
Deduce the wavenumber, in cm−1, of an absorption peak found in the IR spectrum of testosterone but not in that of cholesterol.
-
19N.3.sl.TZ0.13a(i):
State the nuclear equation for the fusion reaction.
- 19N.3.sl.TZ0.16c: Outline how ranitidine reduces stomach acidity.
-
19N.3.sl.TZ0.16a:
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
-
19N.3.hl.TZ0.10a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
-
19N.3.hl.TZ0.12a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
- 19N.3.hl.TZ0.12c: State one similarity and one difference in composition between phospholipids and...
- 19N.3.hl.TZ0.21a: Explain why diamorphine has greater potency than morphine.
- 19N.3.hl.TZ0.14c: Vitamins are organic compounds essential in small amounts. State the name of one functional...
- 19N.3.hl.TZ0.14b: Explain the biomagnification of the pesticide DDT.
- 19N.3.hl.TZ0.25a: Explain how the beta-lactam ring is responsible for the antibiotic properties of penicillin....
-
19N.3.hl.TZ0.18a(i):
State the nuclear equation for the fusion reaction.
- 19N.3.hl.TZ0.18a(ii): Explain why fusion is an exothermic process.
-
19N.3.hl.TZ0.16a:
Discuss the data.
-
19N.3.hl.TZ0.18b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
- 19N.3.hl.TZ0.15c: Explain why maltose, C12H22O11, is soluble in water.
Sub sections and their related questions
A: Materials
- 16N.3.sl.TZ0.3a: Magnesium oxide, MgO, and silicon carbide, SiC, are examples of ceramic materials. State the name...
- 16N.3.sl.TZ0.3b: Predict the predominant type of bonding for a binary compound AB in which the electronegativity...
-
16N.3.sl.TZ0.4a:
Calculate the charge, in coulombs, passed during the electrolysis.
-
16N.3.sl.TZ0.4b:
Calculate the amount, in mol, of electrons passed using section 2 of the data booklet.
-
16N.3.sl.TZ0.4c:
Calculate the mass of indium deposited by one mole of electrons.
-
16N.3.sl.TZ0.4d:
Calculate the number of moles of electrons required to deposit one mole of indium. Relative atomic mass of indium, Ar=114.82.
-
16N.3.sl.TZ0.4e:
Deduce the charge on the indium ion and the formula of indium sulfate.
- 16N.3.sl.TZ0.5a: Explain, with reference to their structure, the great selectivity of zeolites as catalysts.
-
16N.3.sl.TZ0.5b:
Nanocatalysts play an essential role in the manufacture of industrial chemicals.
(i) Describe the high pressure carbon monoxide (HIPCO) method for the production of carbon nanotubes.
(ii) Outline one benefit of using nanocatalysts compared to traditional catalysts in industry.
-
16N.3.sl.TZ0.6a:
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
-
16N.3.sl.TZ0.6b:
Deduce the percentage atom economy for polymerization of 2-methylpropene.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
- 16N.3.sl.TZ0.7a: Outline how a lyotropic liquid crystal differs from a thermotropic liquid crystal.
- 16N.3.sl.TZ0.7b: Explain the effect of increasing the temperature of a nematic liquid crystal on its directional...
-
16N.3.hl.TZ0.6d:
Fermentation of sugars from corn starch produces propane-1,3-diol, which can be polymerized with benzene-1,4-dicarboxylic acid to produce the PTT polymer (polytrimethylene terephthalate).
(i) Draw the molecular structure of each monomer.
(ii) Deduce the name of the linkage formed on polymerization between the two monomers and the name of the inorganic product.
-
16N.3.hl.TZ0.8a:
(i) The diagram below shows the diffraction of two X-ray beams, y and z of wavelength λ, shining on a chromium crystal whose planes are a distance d nm apart.
Deduce the extra distance travelled by the second beam, z, compared to the first one, y.
(ii) State the Bragg’s condition for the observed diffraction to be at its strongest (constructive interference).
-
16N.3.hl.TZ0.8b:
(i) The mass of one unit cell of chromium metal is 17.28 × 10−23 g. Calculate the number of unit cells in one mole of chromium. Ar(Cr) = 52.00.
(ii) Deduce the number of atoms of chromium per unit cell.
- 16N.3.hl.TZ0.9a: Describe the Meissner effect.
- 16N.3.hl.TZ0.9b: Outline one difference between type 1 and type 2 superconductors.
- 16N.3.hl.TZ0.10a: Compare and contrast the Fenton and Haber–Weiss reaction mechanisms.
-
16N.3.hl.TZ0.10b:
Adsorption and chelation are two methods of removing heavy metal ion pollution from the environment.
(i) Describe the process of adsorption.
(ii) Deduce the structure of the complex ion formed by the reaction of three H2N−CH2−CH2−NH2 chelating molecules with a mercury(II) ion.
-
17M.3.sl.TZ1.6a:
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
-
17M.3.sl.TZ1.6b:
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
-
17M.3.sl.TZ1.7a:
State why lanthanum cannot be produced by reducing its oxide with carbon.
-
17M.3.sl.TZ1.7b:
Calculate the current (I), in A, required to produce 1.00 kg of lanthanum metal per hour. Use the formula and sections 2 and 6 of the data booklet.
-
17M.3.sl.TZ1.8a:
State the major advantage that nanoparticles have in these applications.
-
17M.3.sl.TZ1.8b:
Suggest why nanoparticles need to be handled with care.
-
17M.3.sl.TZ1.9a:
Catalysts reduce the activation energy. Outline how homogeneous catalysts are involved in the reaction mechanism.
-
17M.3.sl.TZ1.9b:
Suggest why it is important to know how catalysts function.
-
17M.3.sl.TZ1.9c:
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.sl.TZ1.10a:
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
-
17M.3.sl.TZ1.10b.i:
Describe the difference in their structures.
-
17M.3.sl.TZ1.10b.ii:
Explain why the difference in their structures affects their melting points.
-
17M.3.hl.TZ1.8a:
Lanthanum has a hexagonal close packed (hcp) crystal structure. State the coordination number of each lanthanum atom.
-
17M.3.hl.TZ1.8b:
Lanthanum becomes superconducting below 5 K. Explain, in terms of Bardeen–Cooper–Schrieffer (BCS) theory, how superconductivity occurs.
-
17M.3.hl.TZ1.8c:
Outline why superconductivity only occurs at low temperatures.
-
17M.3.hl.TZ1.9a:
Deduce the repeating unit of the polymer and the other product of the reaction.
-
17M.3.hl.TZ1.9b:
State the class of polymer to which PETE belongs.
-
17M.3.hl.TZ1.10a:
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.hl.TZ1.10b:
Hydrogen sulfide could be used to remove antimony(III) ions from a solution.
Determine the concentration of antimony(III) ions that would be required to precipitate antimony(III) sulfide in a solution saturated with hydrogen sulfide.
[S2−] in water saturated with hydrogen sulfide = 1.0 × 10−14 mol dm−3
Ksp (Sb2S3) = 1.6 × 10−93
-
17M.3.hl.TZ1.10c:
Identify a ligand that could be used to chelate antimony(III) ions in solution.
-
17M.3.sl.TZ2.3a:
State the two distinct phases of a composite.
-
17M.3.sl.TZ2.3b:
Identify the methods of assembling nanocomposites by completing the table.
-
17M.3.sl.TZ2.3c.i:
Explain how the structure of plasticizers enables them to soften PVC.
-
17M.3.sl.TZ2.3c.ii:
Suggest a reason why nanoparticles can better anchor plasticizers in the polymer.
-
17M.3.sl.TZ2.5a:
In a catalytic converter, carbon monoxide is converted to carbon dioxide. Outline the process for this conversion referring to the metal used.
-
17M.3.sl.TZ2.5b.i:
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
-
17M.3.sl.TZ2.5c:
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
-
17M.3.sl.TZ2.6a:
Two important properties of a liquid crystal molecule are being a polar molecule and having a long alkyl chain. Explain why these are essential components of a liquid crystal molecule.
-
17M.3.sl.TZ2.6b:
Metal impurities during the production of LCoS can be analysed using ICP-MS. Each metal has a detection limit below which the uncertainty of data is too high to be valid. Suggest one factor which might influence a detection limit in ICP-MS/ICP-OES.
-
17M.3.hl.TZ2.3c:
Estimate the atom economy of this first step.
-
17M.3.hl.TZ2.3c.ii:
Suggest, giving one reason, whether this is an addition or condensation reaction.
-
17M.3.hl.TZ2.4a:
Identify the other product formed.
-
17M.3.hl.TZ2.4b:
Explain why EDTA, a chelating agent, is more effective in removing heavy metal ions from solution than monodentate ligands.
-
17M.3.hl.TZ2.5b.iii:
Nickel(II) ions are least soluble at pH 10.5. Calculate the molar solubility of nickel(II) hydroxide at this pH. KspNi(OH)2 = 5.48 × 10–16.
-
17M.3.hl.TZ2.5c.i:
Rhodium is paramagnetic with an electron configuration of [Kr] 5s14d8.
Explain, in terms of electron spin pairing, why paramagnetic substances are attracted to a magnetic field and diamagnetic substances are not.
-
17M.3.hl.TZ2.5c.ii:
Rhodium is a type 1 superconductor.
Sketch graphs of resistance against temperature for a conductor and superconductor.

-
17M.3.hl.TZ2.5c.iii:
Contrast type 1 and type 2 superconductors by referring to three differences between them.
-
17N.3.sl.TZ0.4a:
Outline the composition of an alloy and a composite.
- 17N.3.sl.TZ0.4b.i: Outline why an alloy is usually harder than its components by referring to its structure.
- 17N.3.sl.TZ0.4b.ii: At present, composite fillings are more expensive than amalgam fillings. Suggest why a patient...
-
17N.3.sl.TZ0.4c:
Explain how Inductively Coupled Plasma (ICP) Spectroscopy could be used to determine the concentration of mercury in a sample of dental filling.
-
17N.3.sl.TZ0.5:
Catalysts can take many forms and are used in many industrial processes.
Suggest two reasons why it might be worth using a more expensive catalyst to increase the rate of a reaction.
-
17N.3.sl.TZ0.6a:
State equations for the formation of iron nanoparticles and carbon atoms from Fe(CO)5 in the HIPCO process.
- 17N.3.sl.TZ0.6b: Outline why the iron nanoparticle catalysts produced by the HIPCO process are more efficient than...
- 17N.3.sl.TZ0.6c: Discuss one possible risk associated with the use of nanotechnology.
-
17N.3.sl.TZ0.7a:
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
-
17N.3.sl.TZ0.7b.i:
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
-
17N.3.sl.TZ0.7b.ii:
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
-
17N.3.sl.TZ0.7c:
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
-
17N.3.hl.TZ0.6b:
Explain why Type 2 superconductors are generally more useful than Type 1.
- 17N.3.hl.TZ0.7b: Describe how the monomers of addition polymers and of condensation polymers differ.
-
17N.3.hl.TZ0.7c:
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
-
17N.3.hl.TZ0.8a:
Calculate the total number of cobalt atoms within its unit cell.
-
17N.3.hl.TZ0.8b.i:
The atomic radius, r, of cobalt is 1.18 × 10–8 cm. Determine the edge length, in cm, of the unit cell, a, using the second diagram.
-
17N.3.hl.TZ0.8b.ii:
Determine a value for the density of cobalt, in g cm–3, using data from sections 2 and 6 of the data booklet and your answers from (a) and (b) (i).
If you did not obtain an answer to (b) (i), use 3.00 × 10–8 cm but this is not the correct answer.
- 17N.3.hl.TZ0.9a: State the name of one method, other than precipitation, of removing heavy metal ions from...
-
17N.3.hl.TZ0.9b:
The solubility product, Ksp , of cadmium sulfide, CdS, is 8.0 × 10–27. Determine the concentration of cadmium ions in 1.0 dm3 of a saturated solution of cadmium sulfide to which 0.10 mol of solid sodium sulfide has been added, stating any assumption you make.
-
18M.3.hl.TZ1.4c.i:
Draw the structure of the monomer from which nylon-6 is produced by a condensation reaction.
-
18M.3.hl.TZ1.4c.ii:
Deduce, giving a reason, whether the atom economy of a condensation polymerization, such as this, would be greater or less than an addition polymerization, such as the formation of HDPE.
-
18M.3.hl.TZ1.5b:
The diagram illustrates the crystal structure of aluminium metal with the unit cell indicated. Outline the significance of the unit cell.

-
18M.3.hl.TZ1.5c:
When X-rays of wavelength 0.154 nm are directed at a crystal of aluminium, the first order diffraction pattern is observed at 18°. Determine the separation of layers of aluminium atoms in the crystal, in m, using section 1 of the data booklet.
-
18M.3.hl.TZ1.5d.i:
Deduce what the shape of the graph indicates about aluminium.
-
18M.3.hl.TZ1.5d.ii:
Outline why the resistance of aluminium increases above 1.2 K.
-
18M.3.hl.TZ1.5e:
The concentration of aluminium in drinking water can be reduced by precipitating aluminium hydroxide. Calculate the maximum concentration of aluminium ions in water of pH 7 at 298 K. Solubility product of aluminium hydroxide = 3.3 × 10−34 at 298 K.
-
18M.3.hl.TZ2.4a.i:
Deduce the number of atoms per unit cell in vanadium.
-
18M.3.hl.TZ2.4a.ii:
Calculate the expected first order diffraction pattern angle, in degrees, if x-rays of wavelength 150 pm are directed at a crystal of vanadium. Assume the edge length of the crystal to be the same as separation of layers of vanadium atoms found by x-ray diffraction. Use section 1 of the data booklet.
-
18M.3.hl.TZ2.4a.iii:
Calculate the average mass, in g, of a vanadium atom by using sections 2 and 6 of the data booklet.
-
18M.3.hl.TZ2.4a.iv:
Determine the volume, in cm3, of a vanadium unit cell.
-
18M.3.hl.TZ2.4a.v:
Determine the density, in g cm−3, of vanadium by using your answers to (a)(i), (a)(iii) and (a)(iv).
-
18M.3.hl.TZ2.4b.i:
Vanadium and other transition metals can interfere with cell metabolism.
State and explain one process, other than by creating free radicals, by which transition metals interfere with cell metabolism.
-
18M.3.hl.TZ2.4b.ii:
Vanadium(IV) ions can create free radicals by a Fenton reaction.
Deduce the equation for the reaction of V4+ with hydrogen peroxide.
-
18M.3.hl.TZ2.5c.i:
Distinguish between the manufacture of polyester and polyethene.
-
18M.3.hl.TZ2.6b:
MWCNT are very small in size and can greatly increase switching speeds in a liquid crystal allowing the liquid crystal to change orientation quickly.
Discuss two other properties a substance should have to be suitable for use in liquid crystal displays.
-
18M.3.sl.TZ1.3a:
Discuss, in terms of its structure, why an aluminium saucepan is impermeable to water.
-
18M.3.sl.TZ1.3b.i:
State the name given to a material composed of two distinct solid phases.
-
18M.3.sl.TZ1.3b.ii:
State one physical property of HDPE that will be affected by the incorporation of carbon nanotubes.
-
18M.3.sl.TZ1.3b.iii:
Describe how carbon nanotubes are produced by chemical vapour deposition (CVD).
-
18M.3.sl.TZ1.3b.iv:
State the property of carbon nanotubes that enables them to form a nematic liquid crystal phase.
-
18M.3.sl.TZ1.4a:
Both of these are thermoplastic polymers. Outline what this term means.
-
18M.3.sl.TZ1.4b.i:
Compare and contrast the structures of HDPE and LDPE.
-
18M.3.sl.TZ1.4b.ii:
State one way in which a physical property of HDPE, other than density, differs from that of LDPE as a result of this structural difference.
-
18M.3.sl.TZ1.4c.i:
The production of HDPE involves the use of homogeneous catalysts. Outline how homogeneous catalysts reduce the activation energy of reactions.
-
18M.3.sl.TZ1.4c.ii:
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
-
18M.3.sl.TZ1.4d:
Suggest two of the major obstacles, other than collection and economic factors, which have to be overcome in plastic recycling.
-
18M.3.sl.TZ1.4e:
Suggest why there are so many different ways in which plastics can be classified. HDPE can, for example, be categorized thermoplastic, an addition polymer, having Resin Identification Code (RIC) 2, etc.
-
18M.3.sl.TZ1.5:
Aluminium is produced by the electrolysis of a molten electrolyte containing bauxite.
Determine the mass, in g, of aluminium produced by the passage of a charge of 1.296 × 1013 C. Use sections 2 and 6 of the data booklet.
-
18M.3.sl.TZ2.3a:
ICP-OES/MS can be used to analyse alloys and composites. Distinguish between alloys and composites.
-
18M.3.sl.TZ2.3b:
ICP-MS is a reference mode for analysis. The following correlation graphs between ICP-OES and ICP-MS were produced for yttrium and nickel.

Each y-axis shows concentrations calculated by ICP-OES; each x-axis shows concentrations for the same sample as found by ICP-MS.
The line in each graph is y = x.
Discuss the effectiveness of ICP-OES for yttrium and nickel.
-
18M.3.sl.TZ2.3c.i:
Identify the purpose of each graph.
-
18M.3.sl.TZ2.3c.ii:
Calculate, to four significant figures, the concentration, in μg kg−1, of vanadium in oil giving a signal intensity of 14 950.
-
18M.3.sl.TZ2.3c.iii:
Vanadium(V) oxide is used as the catalyst in the conversion of sulfur dioxide to sulfur trioxide.
SO2(g) + V2O5(s) → SO3(g) + 2VO2(s)
O2(g) + 2VO2(s) → V2O5(s)
Outline how vanadium(V) oxide acts as a catalyst.
-
18M.3.sl.TZ2.4a:
Sketch four repeating units of the polymer to show atactic and isotactic polypropene.
-
18M.3.sl.TZ2.4b.i:
State the chemical reason why plastics do not degrade easily.
-
18M.3.sl.TZ2.4b.ii:
Compare two ways in which recycling differs from reusing plastics.
-
18M.3.sl.TZ2.4c:
Civilizations are often characterized by the materials they use.
Suggest an advantage polymers have over materials from the iron age.
-
18M.3.sl.TZ2.5a:
State the source of carbon for MWCNT produced by arc discharge and by CVD.
-
18M.3.sl.TZ2.5b:
Discuss three properties a substance should have to be suitable for use in liquid crystal displays.
-
18N.3.sl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
-
18N.3.sl.TZ0.2b.i:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
-
18N.3.sl.TZ0.2b.ii:
In an incomplete combustion of the polyvinyl chloride, PVC, it was found that hydrogen chloride, carbon monoxide, carbon dioxide, and water vapour were released.
Formulate an equation for this reaction using the formula of the PVC repeating unit.
- 18N.3.sl.TZ0.2c.i: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
- 18N.3.sl.TZ0.2c.ii: Identify another advantage of using a zeolite instead of concentrated sulfuric acid.
-
18N.3.sl.TZ0.2d:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
- 18N.3.sl.TZ0.3a: State the type of particle present in the plasma formed.
-
18N.3.sl.TZ0.3b:
An unknown antacid sample has a lead ion concentration of 0.50 μg dm‒3.
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
18N.3.sl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
- 18N.3.sl.TZ0.4a: Outline two observations that he could have made.
- 18N.3.sl.TZ0.4b: The structure of biphenyl nitrile is shown. Describe, giving a reason, a feature of the...
-
18N.3.sl.TZ0.4c:
Arc discharge, consisting of two inert metal electrodes in a liquid solvent, is one method of producing carbon nanotubes (CNTs).
Predict, giving a reason, the electrode at which the solvent cyclohexane, C6H12, will decompose to form CNTs.
-
18N.3.hl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
-
18N.3.hl.TZ0.2b:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
- 18N.3.hl.TZ0.2c: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
- 18N.3.hl.TZ0.2d.i: State the names of the two terminal functional groups in X.
- 18N.3.hl.TZ0.2d.ii: Deduce the repeating unit of the polymer of X.
-
18N.3.hl.TZ0.2d.iii:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
- 18N.3.hl.TZ0.3a: State the type of particle present in the plasma formed.
-
18N.3.hl.TZ0.3b.i:
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
18N.3.hl.TZ0.3b.ii:
Lead ions are toxic and can be precipitated using hydroxide ions.
Pb2+ (aq) + 2OH‒ (aq) Pb(OH)2 (s)
Sufficient sodium hydroxide solid is added to the antacid sample to produce a 1.0 × 10‒2 mol dm‒3 hydroxide ion solution at 298 K.
Deduce if a precipitate will be formed, using section 32 of the data booklet.
If you did not calculate the concentration of lead ions in (b)(i), use the value of 2.4 × 10−4 mol dm‒3, but this is not the correct value.
-
18N.3.hl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
- 18N.3.hl.TZ0.3d.i: State one feature of a chelating agent.
-
18N.3.hl.TZ0.3d.ii:
An aqueous lead(II) ion reacts with three ethane-1,2-diamine molecules to form an octahedral chelate ion.
Outline why the chelate ion is more stable than the reactants.
- 18N.3.hl.TZ0.5a.i: State the name of the crystal structure of gold.
-
18N.3.hl.TZ0.5a.ii:
Calculate the number of atoms per unit cell of gold, showing your working.
-
18N.3.hl.TZ0.5b:
The edge length of the gold unit cell is 4.08 × 10‒8 cm.
Determine the density of gold in g cm‒3, using sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ1.3a:
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
-
19M.3.hl.TZ1.3b(ii):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.hl.TZ1.3b(iii):
Suggest a better method.
-
19M.3.hl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.hl.TZ1.3d(i):
Lithium has shown some superconductive properties when doped into graphene or when under high pressure. Under high pressure, however, the Meissner effect is absent.
Describe the Meissner effect.
-
19M.3.hl.TZ1.3d(ii):
At very low temperatures, lithium atoms enhance the phonon binding of electrons in graphene suggesting the formation of Cooper pairs.
Explain how Cooper pairs are formed.
-
19M.3.hl.TZ1.3e:
Lithium forms a crystalline lattice with the unit cell structure shown below.
X-ray diffraction shows that the length of the edge of the unit cell is 3.51 × 10−8 cm.
Determine the density of lithium, in g cm−3, using sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.hl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.hl.TZ1.4c:
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.hl.TZ1.4d:
Classify polybutadiene as either an addition or condensation polymer, giving a reason.
-
19M.3.hl.TZ1.4e:
State one factor considered when making green chemistry polymers.
-
19M.3.hl.TZ1.5a:
Label with an asterisk, *, the chiral carbon atom.
-
19M.3.hl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.hl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.hl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.hl.TZ1.7a:
Explain how entropy affects this equilibrium.
-
19M.3.hl.TZ1.7b:
State the number of coordinate covalent bonds EDTA forms with Ni2+.
-
19M.3.hl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase.
Shape of molecules:
Distribution:
-
19M.3.hl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48 250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.hl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19M.3.hl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19M.3.hl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19M.3.hl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.hl.TZ2.5c:
Explain how plasticizers affect the properties of plastics.
-
19M.3.hl.TZ2.5d:
Suggest why the addition of plasticizers is controversial.
-
19M.3.hl.TZ2.5e:
Outline, giving a reason, how addition and condensation polymerization compare with regard to green chemistry.
-
19M.3.hl.TZ2.5f:
Draw the full structural formula of the organic functional group formed during the polymerization of the two reactants below.
-
19M.3.hl.TZ2.6a:
State the number of atoms in the unit cell.
-
19M.3.hl.TZ2.6b:
Determine the density of calcium, in g cm−3, using section 2 of the data booklet.
Ar = 40.08; metallic radius (r) = 1.97 × 10−10 m
-
19M.3.hl.TZ2.7a:
State what is meant by a superconductor.
-
19M.3.hl.TZ2.8a:
Outline why heavy metals are toxic.
-
19M.3.hl.TZ2.8b:
Determine the maximum concentration of lead(II) ions at 298 K in a solution in which the concentration of carbonate ions is maintained at 1.10 × 10−4 mol dm−3. Use section 32 of the data booklet.
-
19M.3.hl.TZ2.8c:
State a method, other than precipitation, of removing heavy metal ions from solution.
-
19M.3.hl.TZ2.18b:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.sl.TZ1.3a(i):
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.sl.TZ1.3a(ii):
Explain why lithium is paramagnetic while lithium hydride is diamagnetic by referring to electron configurations.
-
19M.3.sl.TZ1.3b(i):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.sl.TZ1.3b(ii):
Suggest a better method.
-
19M.3.sl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.sl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.sl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.sl.TZ1.4c(i):
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.sl.TZ1.4c(ii):
The trace quantities of dioxins from tyre fires are rarely inhaled and instead settle on the ground.
Describe why this is a health concern.
-
19M.3.sl.TZ1.5a:
State the name of the functional group which allows the molecule to be responsive to applied electric fields.
-
19M.3.sl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.sl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.sl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.sl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase and the effect that an electric field has on it.
Shape of molecules:Distribution:
Effect of electric field:
-
19M.3.sl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.sl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.sl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19M.3.sl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19M.3.sl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19M.3.sl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.sl.TZ2.5c:
Identify a hazardous product of the incineration of polychloroethene.
-
19M.3.sl.TZ2.5d:
Explain how plasticizers affect the properties of plastics.
-
19M.3.sl.TZ2.5e:
Suggest why the addition of plasticizers is controversial.
-
19N.3.sl.TZ0.3a:
Describe how a heterogeneous catalyst provides an alternative pathway for a reaction.
-
19N.3.sl.TZ0.3c:
Nanotubes are used to support the active material in nanocatalysts.
Explain why oxygen cannot be used for the chemical vapour deposition (CVD) preparation of carbon nanotubes.
- 19N.3.hl.TZ0.4a(i): Outline how resistance to electric currents occurs in metals.
- 19N.3.hl.TZ0.4a(ii): Suggest why the resistance of metals increases with temperature.
- 19N.3.hl.TZ0.4b: State two differences between Type I and Type II superconductors.
-
19N.3.hl.TZ0.5a:
Draw the structure of the monomers of Kevlar® if the by-product of the condensation polymerization is hydrogen chloride.
- 19N.3.hl.TZ0.5b: State and explain why plasticizers are added to polymers.
- 19N.3.hl.TZ0.5c: Discuss why the recycling of plastics is an energy intensive process.
-
19N.3.sl.TZ0.5a:
Discuss why different methods of reduction are needed to extract metals.
-
19N.3.sl.TZ0.5b(i):
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
-
19N.3.sl.TZ0.5b(ii):
Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
-
19N.3.hl.TZ0.7:
X-ray crystallography of a metal crystal produces a diffraction pattern of bright spots.
Using X-rays of wavelength 1.54 × 10−10 m, the first bright spots were produced at an angle θ of 22.3° from the centre.
Calculate the separation between planes of atoms in the lattice, in meters, using section 1 of the data booklet.
-
19N.3.hl.TZ0.8:
1.40 × 10−3 g of NaOH (s) are dissolved in 250.0 cm3 of 1.00 × 10−11 mol dm−3 Pb(OH)2 (aq) solution.
Determine the change in lead ion concentration in the solution, using section 32 of the data booklet.
- 19N.3.sl.TZ0.6a: Describe the arrangement of soap molecules in the nematic liquid crystal phase.
- 19N.3.sl.TZ0.6b: State how liquid crystals are affected by an electric field.
- 19N.3.sl.TZ0.3b: Distinguish between heterogeneous and homogeneous catalysts, giving one difference.
-
19N.3.sl.TZ0.4a:
Draw a section of an isotactic polypropene polymer chain containing four repeating units.
- 19N.3.sl.TZ0.4b: Predict, with a reason, whether isotactic or atactic polypropene has the higher melting point.
- 19N.3.sl.TZ0.4c: Polypropene is a thermoplastic. Outline what is meant by thermoplastic.
- 19N.3.sl.TZ0.4d: Discuss why the recycling of plastics is an energy intensive process.
-
20N.3.sl.TZ0.3a:
Outline the two distinct phases of this composite.
- 20N.3.sl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.sl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
- 20N.3.sl.TZ0.3c: Explain why phthalates are replaced by other plasticizers in the production of plastics.
- 20N.3.sl.TZ0.4a: Explain these properties of carbon nanotubes.
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
-
20N.3.sl.TZ0.4b(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020.
Write the half-equations for the reactions occurring in this electrolysis.
-
20N.3.sl.TZ0.4b(iii):
Calculate the theoretical mass of magnesium obtained if a current of is used for hours. Use charge and section 2 of the data booklet
-
20N.3.sl.TZ0.4b(iv):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
- 20N.3.sl.TZ0.4c: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
- 20N.3.sl.TZ0.4d: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
-
20N.3.hl.TZ0.3a:
Outline the two distinct phases of this composite.
- 20N.3.hl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.hl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
- 20N.3.hl.TZ0.3b(iii): Explain why phthalates are replaced by other plasticizers in the production of plastics.
- 20N.3.hl.TZ0.3c: Classify PVC and polyethene terephthalate, PET, as addition or condensation polymers and deduce...
- 20N.3.hl.TZ0.4a: Explain these properties of carbon nanotubes.
- 20N.3.hl.TZ0.4b(i): CNT can act as Type 2 superconductors. Outline why Type 2 superconductors are generally more...
-
20N.3.hl.TZ0.4b(ii):
Explain the role of electrons in superconducting materials in terms of the Bardeen–Cooper–Schrieffer (BCS) theory.
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
-
20N.3.hl.TZ0.4c(iii):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
- 20N.3.hl.TZ0.4d: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
-
20N.3.hl.TZ0.5a:
Precipitation is one method used to treat waste water.
Phosphates, , in waste water can be removed by precipitation with magnesium ions. of magnesium phosphate is .
Calculate the maximum solubility of phosphate ions in a solution containing magnesium ions.
-
20N.3.hl.TZ0.4c(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020
Calculate the theoretical mass of magnesium obtained if a current of 3.00 A is used for hours. Use charge :(Q) = current (I) × time (t) and section 2 of the data booklet.
-
20N.3.hl.TZ0.5b:
Precipitation is one method used to treat waste water.
Zinc, cadmium, nickel, and lead are metal ions which can be removed by precipitation. Explain why waste water is adjusted to a pH of 9−10 to remove these ions by referring to section 32 of the data booklet.
- 20N.3.hl.TZ0.4e: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
B: Biochemistry
- 16N.3.sl.TZ0.8a: Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed...
-
16N.3.sl.TZ0.8b:
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
- 16N.3.sl.TZ0.9a: State the raw materials and source of energy used in the process described above.
-
16N.3.sl.TZ0.9b:
The structures of two molecules, X and Y, are shown below.
(i) Justify why both these molecules are carbohydrates.
(ii) Distinguish between these molecules in terms of their functional groups.
-
16N.3.sl.TZ0.9c:
Amylose is an unbranched polysaccharide composed of repeating units of glucose.
(i) Draw the structure of the repeating unit of amylose. Use section 34 of the data booklet.
(ii) Amylose is a major component of starch. Corn starch can be used to make replacements for plastics derived from oil, especially for packaging. Discuss one potential advantage and one disadvantage of this use of starch.
-
16N.3.sl.TZ0.10b:
A mixture of amino acids is separated by gel electrophoresis at pH 6.0. The amino acids are then stained with ninhydrin.
(i) On the diagram below draw the relative positions of the following amino acids at the end of the process: Val, Asp, Lys and Thr.
(ii) Suggest why glycine and isoleucine separate slightly at pH 6.5.
- 16N.3.sl.TZ0.10c: Determine the number of different tripeptides that can be made from twenty different amino acids.
-
16N.3.sl.TZ0.10d:
The fibrous protein keratin has a secondary structure with a helical arrangement.
(i) State the type of interaction responsible for holding the protein in this arrangement.
(ii) Identify the functional groups responsible for these interactions.
-
16N.3.hl.TZ0.13c:
Amino acids act as buffers in solution. In aspartic acid, the side chain (R group) carboxyl has pKa = 4.0. Determine the percentage of the side chain carboxyl that will be ionized (–COO–) in a solution of aspartic acid with pH = 3.0. Use section 1 of the data booklet.
-
16N.3.hl.TZ0.14a:
(i) Estimate the Km values of the two enzymes.
(ii) Suggest, with a reason, which enzyme will be more responsive to changes in the concentration of glucose in the blood.
-
16N.3.hl.TZ0.14b:
(i) Outline what is meant by product inhibition as it applies to hexokinase.
(ii) Product inhibition of hexokinase does not affect its Km value. Using this information, deduce the type of binding site that the inhibitor attaches to.
- 16N.3.hl.TZ0.15a: State the name of the component of DNA responsible for the migration of its fragments to the...
-
16N.3.hl.TZ0.15b:
In 2010, scientists claimed that they had discovered a species of bacteria capable of incorporating arsenic in place of phosphorus into the bacterial DNA. This claim has since proved controversial. Suggest one technique or evidence that might help support the claim.
- 16N.3.hl.TZ0.16a: Outline why this molecule absorbs visible light.
- 16N.3.hl.TZ0.16b: With reference to its chemical structure, outline whether this pigment is found in aqueous...
-
16N.3.hl.TZ0.16c:
A student investigated the ability of anthocyanins to act as pH indicators. He extracted juice from blackberries and used a UV-vis spectrophotometer to produce absorption spectra at different pH values. His results are shown below.
Deduce the colour of the juice at each pH, giving your reasoning. Use section 17 of the data booklet.
-
17M.3.sl.TZ1.11a:
List the building blocks of triglycerides and carbohydrates.
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ1.11b.ii:
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
-
17M.3.sl.TZ1.11c:
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16. -
17M.3.sl.TZ1.12a.i:
Deduce the straight chain structure of ribose from its ring structure drawn in section 34 of the data booklet.
-
17M.3.sl.TZ1.12a.ii:
Using the partial structure given, complete the structural formula of the molecule formed from the condensation of two cyclic -glucose molecules.

-
17M.3.sl.TZ1.12b:
Constructing models that allow visualizations of the stereochemistry of carbohydrates is essential to understand their structural roles in cells.
Describe how Haworth projections help focus on the position of attached groups.
-
17M.3.sl.TZ1.12c.i:
State one advantage of starch based polymers besides being biodegradable.
-
17M.3.sl.TZ1.12c.ii:
Biodegradable boxes made from polylactic acid, PLA, disintegrate when exposed to water.
State the formula of the product formed when water reacts with PLA.
-
17M.3.sl.TZ1.13a:
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
-
17M.3.sl.TZ1.13b.i:
Identify the name of the amino acid that does not move under the influence of the applied voltage.
-
17M.3.sl.TZ1.13b.ii:
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
-
17M.3.sl.TZ1.13c:
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
-
17M.3.sl.TZ1.13d:
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
-
17M.3.sl.TZ1.13e:
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
-
17M.3.sl.TZ1.13f:
Outline how lead ions could be removed from an individual suffering from lead poisoning.
-
17M.3.hl.TZ1.15a:
Deduce the pH range in which glycine is an effective buffer in basic solution.
-
17M.3.hl.TZ1.15b:
Enzymes are biological catalysts.
The data shows the effect of substrate concentration, [S], on the rate, v, of an enzyme-catalysed reaction.
Determine the value of the Michaelis constant (Km) from the data. A graph is not required.
-
17M.3.hl.TZ1.15c:
Outline the action of a non-competitive inhibitor on the enzyme-catalysed reaction.
-
17M.3.hl.TZ1.15d:
The sequence of nitrogenous bases in DNA determines hereditary characteristics.
Calculate the mole percentages of cytosine, guanine and thymine in a double helical DNA structure if it contains 17% adenine by mole.
-
17M.3.hl.TZ1.16a:
Explain the shape of the curve at low oxygen partial pressure up to about 5 kPa.
-
17M.3.hl.TZ1.16b.i:
Sketch a graph on the axes above to show the effect of decreasing pH on the binding of oxygen to hemoglobin (the Bohr Effect).
-
17M.3.hl.TZ1.16b.ii:
Outline the effect of decreasing pH on the oxygen saturation of hemoglobin.
-
17M.3.hl.TZ1.17:
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
-
17M.3.sl.TZ2.7a:
Deduce the structural formula of the dipeptide Cys-Lys.
-
17M.3.sl.TZ2.7b:
Identify the type of bond between two cysteine residues in the tertiary structure of a protein.
-
17M.3.sl.TZ2.7c:
Deduce the structural formula of the predominant form of cysteine at pH 1.0.
-
17M.3.sl.TZ2.7d:
A mixture of the three amino acids, cysteine, glutamine and lysine, was placed in the centre of a square plate covered in polyacrylamide gel. The gel was saturated with a buffer solution of pH 6.0. Electrodes were connected to opposite sides of the gel and a potential difference was applied.
Sketch lines on the diagram to show the relative positions of the three amino acids after electrophoresis.

-
17M.3.sl.TZ2.8a:
Explain which one of these fatty acids has the highest boiling point.
-
17M.3.sl.TZ2.8b:
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 moldm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
-
17M.3.sl.TZ2.9a:
Glycerol is one product of the reaction. Identify the two other organic products.
-
17M.3.sl.TZ2.9b:
Identify the type of reaction which occurs.
-
17M.3.sl.TZ2.10a:
Identify the functional groups which are present in only one structure of glucose.
-
17M.3.sl.TZ2.10b:
Sucrose is a disaccharide formed from -glucose and β-fructose.
Deduce the structural formula of sucrose.
-
17M.3.sl.TZ2.10c:
Starch is a constituent of many plastics. Suggest one reason for including starch in plastics.
-
17M.3.sl.TZ2.10d:
Suggest one of the challenges scientists face when scaling up the synthesis of a new compound.
-
17M.3.sl.TZ2.11:
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
-
17M.3.hl.TZ2.8c.i:
An aqueous buffer solution contains both the zwitterion and the anionic forms of alanine. Draw the zwitterion of alanine.
-
17M.3.hl.TZ2.8c.ii:
Calculate the pH of a buffer solution which contains 0.700 mol dm–3 of the zwitterion and 0.500 mol dm–3 of the anionic form of alanine.
Alanine pKa = 9.87.
-
17M.3.hl.TZ2.12a:
Identify the structural feature which enables rhodopsin to absorb visible light.
-
17M.3.hl.TZ2.12b:
Outline the change that occurs in the retinal residue during the absorption of visible light.
-
17M.3.hl.TZ2.13a:
Determine the value of the Michaelis constant, Km, including units, from the graph.
-
17M.3.hl.TZ2.13b:
Sketch a second graph on the same axes to show how the reaction rate varies when a competitive inhibitor is present.
-
17M.3.hl.TZ2.13c:
Outline the significance of the value of Km.
-
17M.3.hl.TZ2.14a:
Explain the shape of the curve from 0 to X kPa.
-
17M.3.hl.TZ2.14b:
Explain why carbon monoxide is toxic to humans.
-
17M.3.hl.TZ2.15a:
Outline how its structure allows it to be negatively charged in the body.
-
17M.3.hl.TZ2.15b:
Deduce the nucleotide sequence of a complementary strand of a fragment of DNA with the nucleotide sequence –GACGGATCA–.
- 17N.3.sl.TZ0.8a.ii: The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per...
- 17N.3.sl.TZ0.8b.i: State the type of reaction occurring during the titration.
-
17N.3.sl.TZ0.8b.ii:
Calculate the volume of iodine solution used to reach the end-point.
-
17N.3.sl.TZ0.8c:
Outline the importance of linoleic acid for human health.
-
17N.3.sl.TZ0.9a:
Describe what is meant by a condensation reaction.
- 17N.3.sl.TZ0.9b: Draw the structure of galactose on the skeleton provided.
-
17N.3.sl.TZ0.9c:
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
-
17N.3.sl.TZ0.10a:
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
- 17N.3.sl.TZ0.10b: State one function of vitamin D in the body.
- 17N.3.sl.TZ0.11: Enzyme activity depends on many factors. Explain how pH change causes loss of activity of an enzyme.
-
17N.3.hl.TZ0.11a:
Determine the value of the Michaelis constant, Km, by annotating the graph.
-
17N.3.hl.TZ0.11b.i:
The malonate ion acts as an inhibitor for the enzyme.
Suggest, on the molecular level, how the malonate ion is able to inhibit the enzyme.
-
17N.3.hl.TZ0.11b.ii:
Draw a curve on the graph above showing the effect of the presence of the malonate ion inhibitor on the rate of reaction.
-
17N.3.hl.TZ0.13:
The stability of DNA is due to interactions of its hydrophilic and hydrophobic components.
Outline the interactions of the phosphate groups in DNA with water and with surrounding proteins (histones).
-
17N.3.hl.TZ0.14a:
State the half-equation for the reduction of molecular oxygen to water in acidic conditions.
- 17N.3.hl.TZ0.14b: Outline the change in oxidation state of the iron ions in heme groups that occurs when molecular...
-
17N.3.hl.TZ0.15b:
Retinal is the key molecule involved in vision. Explain the roles of cis and trans-retinal in vision and how the isomers are formed in the visual cycle.
-
18M.3.hl.TZ1.6d:
Describe how DNA determines the primary structure of a protein such as insulin.
-
18M.3.hl.TZ1.8b:
Outline why cellulose fibres are strong.
-
18M.3.hl.TZ1.9a:
Explain with reference to the binding site on the enzyme how a non-competitive inhibitor lowers the value of Vmax.
-
18M.3.hl.TZ1.9b:
Outline the significance of the value of the Michaelis constant, Km.
-
18M.3.hl.TZ1.10a:
Outline why anthocyanins are coloured.
-
18M.3.hl.TZ1.10b:
Explain why the blue colour of a quinoidal base changes to the red colour of a flavylium cation as pH decreases.
-
18M.3.hl.TZ2.8c:
Draw the structures of the main form of glycine in buffer solutions of pH 1.0 and 6.0.
The pKa of glycine is 2.34.
-
18M.3.hl.TZ2.8d:
Calculate the pH of a buffer system with a concentration of 1.25 × 10−3 mol dm−3 carbonic acid and 2.50 × 10−2 mol dm−3 sodium hydrogen carbonate. Use section 1 of the data booklet.
pKa (carbonic acid) = 6.36
-
18M.3.hl.TZ2.10b:
Explain how the structure of vitamin A is important to vision using section 35 of the data booklet.
-
18M.3.hl.TZ2.11a:
Hemoglobin’s oxygen dissociation curve is shown at a given temperature. Sketch the curve on the graph at a higher temperature.

-
18M.3.hl.TZ2.11b:
Outline two differences between normal hemoglobin and foetal hemoglobin.
-
18M.3.hl.TZ2.12:
DNA is a biopolymer made up of nucleotides. List two components of a nucleotide.
-
18M.3.sl.TZ1.6a:
Draw the structural formula of a dipeptide containing the residues of valine, Val, and asparagine, Asn, using section 33 of the data booklet.
-
18M.3.sl.TZ1.6b:
Deduce the strongest intermolecular forces that would occur between the following amino acid residues in a protein chain.
-
18M.3.sl.TZ1.6c.i:
State the name of the process used to break down the insulin protein into its constituent amino acids.
-
18M.3.sl.TZ1.6c.ii:
Outline how the amino acids may be identified from a paper chromatogram.
-
18M.3.sl.TZ1.7a.i:
Identify the type of rancidity occurring in saturated lipids and the structural feature that causes it.
-
18M.3.sl.TZ1.7a.ii:
State one factor that increases the rate at which saturated lipids become rancid.
-
18M.3.sl.TZ1.7b:
Butter contains varying proportions of oleic, myristic, palmitic and stearic acids. Explain in terms of their structures why stearic acid has a higher melting point than oleic acid, using section 34 of the data booklet.
-
18M.3.sl.TZ1.7c.i:
Fish oil is an excellent dietary source of omega-3 fatty acids. Outline one impact on health of consuming omega-3 fatty acids.
-
18M.3.sl.TZ1.7c.ii:
Predict the solubility of retinol (vitamin A) in body fat, giving a reason. Use section 35 of the data booklet.
-
18M.3.sl.TZ1.7c.iii:
Explain why sharks and swordfish sometimes contain high concentrations of mercury and polychlorinated biphenyls (PCBs).
-
18M.3.sl.TZ1.7c.iv:
Plastics are another source of marine pollution. Outline one way in which plastics can be made more biodegradable.
-
18M.3.sl.TZ1.8a:
State the specific type of linkage formed between α-glucose fragments in both maltose and amylose.
-
18M.3.sl.TZ1.8b:
A person with diabetes suffering very low blood sugar (hypoglycaemia) may be advised to consume glucose immediately and then eat a small amount of starchy food such as a sandwich. Explain this advice in terms of the properties of glucose and starch.
-
18M.3.sl.TZ2.6a:
Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
-
18M.3.sl.TZ2.6b:
Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon–carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid Mr = 304.5)
-
18M.3.sl.TZ2.6c:
Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
-
18M.3.sl.TZ2.6d:
Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
-
18M.3.sl.TZ2.6e:
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
-
18M.3.sl.TZ2.6f:
Explain why lipids provide more energy than carbohydrates and proteins.
-
18M.3.sl.TZ2.7a:
Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
-
18M.3.sl.TZ2.7c:
Outline why amino acids have high melting points.
-
18M.3.sl.TZ2.8:
Green Chemistry reduces the production of hazardous materials and chemical waste.
Outline two specific examples or technological processes of how Green Chemistry has accomplished this environmental impact.
-
18M.3.sl.TZ2.9:
Explain the solubility of vitamins A and C using section 35 of the data booklet.
-
18M.3.hl.TZ2.8e:
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
-
18M.3.hl.TZ2.8f:
UV-Vis spectroscopy can be used to determine the unknown concentration of a substance in a solution.
Calculate the concentration of an unknown sample of pepsin with an absorbance of 0.725 using section 1 of the data booklet.
Cell length = 1.00 cm
Molar absorptivity (extinction coefficient) of the sample = 49650 dm3 cm−1 mol−1
-
18M.3.hl.TZ2.8g:
A different series of pepsin samples is used to develop a calibration curve.
Estimate the concentration of an unknown sample of pepsin with an absorbance of 0.30 from the graph.
- 18N.3.sl.TZ0.5a: The formation of proteins from amino acids is an example of an anabolic reaction in the human...
-
18N.3.sl.TZ0.5b:
Suggest why it is advisable for those living in northerly or southerly latitudes (that is away from the equator) to take vitamin D supplements during the winter.
- 18N.3.sl.TZ0.5c: Explain how a xenobiotic is biomagnified.
- 18N.3.sl.TZ0.6a: Describe the interaction responsible for the secondary structure of a protein.
- 18N.3.sl.TZ0.6b.i: Explain the action of an enzyme and state one of its limitations.
- 18N.3.sl.TZ0.6b.ii: Enzymes are widely used in washing detergents. Outline how they improve the efficiency of the...
- 18N.3.sl.TZ0.7a: A phospholipid generally consists of two hydrophobic fatty acids and a hydrophilic...
-
18N.3.sl.TZ0.7b.i:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
- 18N.3.sl.TZ0.7b.ii: State two functions of lipids in the body.
- 18N.3.sl.TZ0.7c: Outline one effect of increased levels of low-density lipoproteins in the blood.
- 18N.3.sl.TZ0.8a: Name the type of link between the two monosaccharide residues.
- 18N.3.sl.TZ0.8b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.hl.TZ0.7a: State the feature of DNA that determines the primary structure of proteins synthesised by a cell.
- 18N.3.hl.TZ0.7b: Suggest one concern about the use of genetically modified, GM, food.
- 18N.3.hl.TZ0.8a: Describe the interaction responsible for the secondary structure of a protein.
- 18N.3.hl.TZ0.8b: Explain the action of an enzyme and state one of its limitations.
-
18N.3.hl.TZ0.8c:
Contrast the actions of non-competitive and competitive inhibitors of an enzyme and state their effects on the maximum rate of reaction, Vmax, and the Michaelis–Menten constant, Km.
- 18N.3.hl.TZ0.9b: State two functions of lipids in the body.
- 18N.3.hl.TZ0.9c: Outline one effect of increased levels of low-density lipoproteins in the blood.
-
18N.3.hl.TZ0.9a:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
- 18N.3.hl.TZ0.10a: Name the type of link between the two monosaccharide residues.
- 18N.3.hl.TZ0.10b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.hl.TZ0.10c.i: Outline the difference between their structures.
- 18N.3.hl.TZ0.10c.ii: Outline why cellulose is an essential part of human diet.
- 18N.3.hl.TZ0.11a: A graph showing saturation of oxygen against partial pressure of oxygen is shown. Explain the...
-
18N.3.hl.TZ0.11b:
Explain why carbon monoxide is very toxic and how it may be possible to treat carbon monoxide poisoning.
-
19M.3.hl.TZ1.8a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.hl.TZ1.8b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.hl.TZ1.8c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.hl.TZ1.8d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.hl.TZ1.9a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.hl.TZ1.9b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.hl.TZ1.9c:
Aspartic acid is obtained synthetically as a racemic mixture. Draw the three‑dimensional shape of each isomer showing their spatial relationship to each other. Use section 33 of the data booklet.
-
19M.3.hl.TZ1.10a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.hl.TZ1.10b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.hl.TZ1.10c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline one effect of trans-fatty acids on health.
-
19M.3.hl.TZ1.11a:
The absorption spectrum of β-carotene is shown below.
Explain its colour in terms of its absorption bands. Use section 17 of the data booklet.
-
19M.3.hl.TZ1.11b:
The absorption spectrum of chlorophyll a is shown below.
Suggest how the combination of chlorophyll a and carotenoids is beneficial for photosynthesis.
-
19M.3.hl.TZ1.12a(i):
A Michaelis–Menten plot for an enzyme-catalysed reaction is shown.
Sketch a curve to show the effect of a competitive inhibitor.
-
19M.3.hl.TZ1.12a(ii):
Suggest, based on the Michaelis–Menten plot, how a competitive inhibitor such as ethanol reduces the toxicity of methanol.
-
19M.3.hl.TZ1.12b:
Enzymatic activity is studied in buffered aqueous solutions.
Calculate the ratio in which 0.1 mol dm−3 NaH2PO4 (aq) and 0.1 mol dm−3 Na2HPO4 (aq) should be mixed to obtain a buffer with pH = 6.10. Use section 1 of the data booklet.
pKa (NaH2PO4) = 7.20
-
19M.3.hl.TZ1.13:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.hl.TZ1.14a:
Outline what is meant by genetically modified organisms.
-
19M.3.hl.TZ1.14b:
Outline one benefit of the use of these products.
-
19M.3.hl.TZ1.19b(i):
Deduce the protons responsible for signals X and Y by marking them on the structure of aspirin in (a). Use section 27 of the data booklet.
-
19M.3.hl.TZ1.19b(ii):
Identify the splitting pattern of signals X and Y.
X:
Y:
-
19M.3.hl.TZ2.9a(i):
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.hl.TZ2.9a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.hl.TZ2.9b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19M.3.hl.TZ2.9c:
State and explain how a competitive inhibitor affects the maximum rate, Vmax, of an enzyme-catalyzed reaction.
-
19M.3.hl.TZ2.9d(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.hl.TZ2.9d(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
-
19M.3.hl.TZ2.10a(i):
Outline which pKa value should be used when calculating the pH of the solution, giving your reason.
-
19M.3.hl.TZ2.10a(ii):
Calculate the pH of the glutamine solution using section 1 of the data booklet.
-
19M.3.hl.TZ2.10b:
Describe what is meant by the genetic code and how it relates to protein synthesis.
-
19M.3.hl.TZ2.11a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.hl.TZ2.11a(ii):
Identify the type of reaction in (a).
-
19M.3.hl.TZ2.11b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.hl.TZ2.11c:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
-
19M.3.hl.TZ2.11d:
Phospholipids are also found in lipoprotein structures.
Describe one effect of increased levels of low-density lipoprotein (LDL) on health.
-
19M.3.hl.TZ2.12a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.hl.TZ2.12b:
Classify, giving your reason, the hexose (six-membered) ring of sucrose as an α or β isomer.
-
19M.3.hl.TZ2.12c:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19M.3.hl.TZ2.13a:
Outline why the complex formed between Fe2+ and oxygen is red. Refer to the diagram above and section 17 of the data booklet.
-
19M.3.hl.TZ2.13b(i):
Explain the shape of the curve.
- 19M.3.hl.TZ2.13b(ii): Sketch another line to show the effect of an increase in body temperature on the oxygen...
-
19M.3.sl.TZ1.7a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.sl.TZ1.7b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.sl.TZ1.7c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.sl.TZ1.7d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.sl.TZ1.8a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.sl.TZ1.8b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.sl.TZ1.9a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.sl.TZ1.9b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.sl.TZ1.9c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline two effects of trans-fatty acids on health.
-
19M.3.sl.TZ1.10:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.sl.TZ2.6a(i) :
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.sl.TZ2.6a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.sl.TZ2.6b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19M.3.sl.TZ2.6c(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.sl.TZ2.6c(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
-
19M.3.sl.TZ2.7a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.sl.TZ2.7a(ii):
Identify the type of reaction in (a).
-
19M.3.sl.TZ2.7b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.sl.TZ2.7c:
Predict, giving a reason, the relative energy density of a carbohydrate and a lipid of similar molar mass.
-
19M.3.sl.TZ2.7d:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
-
19M.3.sl.TZ2.7e:
Phospholipids are also found in lipoprotein structures.
Describe two effects of increased levels of low-density lipoprotein (LDL) on health.
-
19M.3.sl.TZ2.8a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.sl.TZ2.8b:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19N.3.sl.TZ0.7a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
- 19N.3.hl.TZ0.10b(i): Describe, using another method, how a mixture of four amino acids, alanine, arginine, glutamic...
- 19N.3.hl.TZ0.10b(ii): Suggest why alanine and glycine separate slightly at pH 6.5.
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
- 19N.3.hl.TZ0.11a: Outline the significance of the Michaelis constant Km.
-
19N.3.hl.TZ0.11b:
Compare the effects of competitive and non-competitive inhibitors.
-
19N.3.sl.TZ0.9a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
-
19N.3.hl.TZ0.12b:
The chemical change in stored fats causes rancidity characterized by an unpleasant smell or taste.
Compare hydrolytic and oxidative rancidity.
- 19N.3.sl.TZ0.9d(i): State one similarity and one difference in composition between phospholipids and...
-
19N.3.hl.TZ0.13a:
List two components of nucleotides.
- 19N.3.hl.TZ0.13b: Explain how the double-helical structure of DNA is stabilized once formed.
- 19N.3.hl.TZ0.14a: The graph shows the change in oxygen partial pressure in blood, measured at different pH...
- 19N.3.sl.TZ0.10b: Explain the biomagnification of the pesticide DDT.
- 19N.3.sl.TZ0.10a: State the name of one functional group common to all three vitamins shown in section 35 of the...
- 19N.3.hl.TZ0.15a: Describe the function of chlorophyll in photosynthesis.
- 19N.3.hl.TZ0.15b: Compare and contrast the structures of starch and cellulose. One similarity: One difference:
-
19N.3.sl.TZ0.7b:
The isoelectric point of amino acids is the intermediate pH at which an amino acid is electrically neutral.
Suggest why Asp and Phe have different isoelectric points.
- 19N.3.sl.TZ0.8a: The graph shows the relationship between the temperature and the rate of an enzyme-catalysed...
-
19N.3.sl.TZ0.8b:
Explain why a change in pH affects the tertiary structure of an enzyme in solution.
- 19N.3.sl.TZ0.8c: State one use of enzymes in reducing environmental problems.
- 19N.3.sl.TZ0.9b: State one impact on health of the increase in LDL cholesterol concentration in blood.
- 19N.3.sl.TZ0.9c: Explain why stearic acid has a higher melting point than oleic acid.
-
19N.3.sl.TZ0.9d(ii):
Identify a reagent that hydrolyses triglycerides.
- 19N.3.sl.TZ0.10c: Explain why maltose, C12H22O11, is soluble in water.
-
19N.3.hl.TZ0.10a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
-
19N.3.hl.TZ0.12a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
- 19N.3.hl.TZ0.12c: State one similarity and one difference in composition between phospholipids and...
- 19N.3.hl.TZ0.14b: Explain the biomagnification of the pesticide DDT.
- 19N.3.hl.TZ0.14c: Vitamins are organic compounds essential in small amounts. State the name of one functional...
- 19N.3.hl.TZ0.15c: Explain why maltose, C12H22O11, is soluble in water.
-
20N.3.sl.TZ0.5a(i):
Proteins are polymers of amino acids. A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
-
20N.3.sl.TZ0.5a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.sl.TZ0.5b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
-
20N.3.sl.TZ0.5c:
Proteins are polymers of amino acids.
Describe how the tertiary structure differs from the quaternary structure in hemoglobin.
-
20N.3.sl.TZ0.6a:
Deduce the products of the hydrolysis of a non-substituted phospholipid, where and represent long alkyl chains.
-
20N.3.sl.TZ0.6b(i):
A representation of a phospholipid bilayer cell membrane is shown:
© International Baccalaureate Organization 2020.
Identify the components of the phospholipid labelled A and B.
-
20N.3.sl.TZ0.6c:
Phospholipids help maintain cellular environments while fatty acid lipids have important roles in energy storage and electrical insulation. Discuss the structural properties of saturated fats needed for these roles.
- 20N.3.sl.TZ0.7a: The diverse functions of biological molecules depend on their structure and shape. Classify...
-
20N.3.sl.TZ0.7b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
-
20N.3.sl.TZ0.7b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Sucrose is a disaccharide formed in the reaction of glucose with fructose.
Identify the reaction type and the newly formed functional group that joins the monosaccharide units in the product.
-
20N.3.sl.TZ0.8a:
Calculate the BMF if a shark consumes mackerel in one year. Each mackerel weighs on average. The per body weight. Assume chemical remains in the shark’s body for two years.
- 20N.3.sl.TZ0.8b: Suggest, with a reason, if fat-soluble or water-soluble xenobiotics would have a larger BMF.
-
20N.3.hl.TZ0.6a(i):
Proteins are polymers of amino acids.
A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
-
20N.3.hl.TZ0.6a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.hl.TZ0.6b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
- 20N.3.hl.TZ0.6c(i): Proteins are polymers of amino acids. Sketch and label two oxygen dissociation curves, one for...
-
20N.3.hl.TZ0.6c(ii):
Proteins are polymers of amino acids.
Explain why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin.
- 20N.3.hl.TZ0.8a: The diverse functions of biological molecules depend on their structure and shape. Classify...
-
20N.3.hl.TZ0.8b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
-
20N.3.hl.TZ0.8b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Draw the nitrogenous base that is paired with guanine in DNA, showing the hydrogen bonds between the bases. Use section 34 of the data booklet.
- 20N.3.hl.TZ0.8c: The diverse functions of biological molecules depend on their structure and shape. Retinal is...
- 20N.3.hl.TZ0.10a: Identify the type of inhibition shown in the graph.
-
20N.3.hl.TZ0.10b(i):
Determine the value of and in the absence and presence of the inhibitor.
-
20N.3.hl.TZ0.10b(ii):
Outline the significance of the value of the Michaelis constant, .
C: Energy
-
16N.3.sl.TZ0.11a:
(i) Calculate the specific energy of the lithium ion battery, in MJ kg−1, when 80.0 kg of fuel in the battery releases 1.58 × 107 J. Use section 1 of the data booklet.
(ii) The specific energy of gasoline is 46.0 MJ kg−1. Suggest why gasoline may be considered a better energy source than the lithium ion battery based on your answer to part (a) (i).
-
16N.3.sl.TZ0.11b:
(i) The energy density of gasoline is 34.3 MJ dm−3. Calculate the volume of gasoline, in dm3, that is equivalent to the energy in 80.0 kg of fuel in the lithium ion battery. Use section 1 of the data booklet.
(ii) The efficiency of energy transfer by this lithium ion battery is four times greater than that of gasoline. Determine the distance, in km, the car can travel on the lithium ion battery power alone if the gasoline-powered car uses 1.00 dm3 gasoline to travel 32.0 km.
- 16N.3.sl.TZ0.12a: Discuss how the octane number changes with the molecular structure of the alkanes.
- 16N.3.sl.TZ0.12b: Catalytic reforming and cracking reactions are used to produce more efficient fuels. Deduce the...
-
16N.3.sl.TZ0.13a:
Explain the effect of the increasing concentration of atmospheric carbon dioxide on the acidity of oceans.
-
16N.3.sl.TZ0.13b:
(i) Describe the changes that occur at the molecular level when atmospheric carbon dioxide gas absorbs infrared radiation emitted from the Earth’s surface.
(ii) Other than changes to the acidity of oceans, suggest why the production of carbon dioxide is of greater concern than the production of water vapour.
- 16N.3.sl.TZ0.14a: State the equation for the complete transesterification of the triglyceride given below with...
-
16N.3.sl.TZ0.14b:
Outline why the fuel produced by the reaction in (a) is more suitable for use in diesel engines than vegetable oils.
-
16N.3.sl.TZ0.15a:
(i) Explain why fusion, combining two smaller nuclei into a larger nucleus, releases vast amounts of energy. Use section 36 of the data booklet.
(ii) Outline one advantage of fusion as a source of energy.
-
16N.3.sl.TZ0.15b:
Radioactive phosphorus, 33P, has a half-life of 25.3 days.
(i) Calculate 33P decay constant λ and state its unit. Use section 1 of the data booklet.
(ii) Determine the fraction of the 33P sample remaining after 101.2 days.
-
16N.3.hl.TZ0.21a:
The Geobacter species of bacteria can be used in microbial fuel cells to oxidise aqueous ethanoate ions,
CH3COO−(aq), to carbon dioxide gas.State the half-equations for the reactions at both electrodes.
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
-
16N.3.hl.TZ0.21c:
Dye-sensitized solar cells (DSSC) convert solar energy into electrical energy.
(i) Describe how a DSSC converts sunlight into electrical energy.
(ii) Explain the role of the electrolyte solution containing iodide ions, I−, and triiodide ions, I3−, in the DSSC.
-
17M.3.sl.TZ1.14a:
Outline how the spectra of light from stars can be used to detect the presence of carbon.
-
17M.3.sl.TZ1.14b.i:
Deduce the identity of X.
-
17M.3.sl.TZ1.14b.ii:
Outline why this reaction results in a release of energy.
-
17M.3.sl.TZ1.14c:
Nuclear fusion reactors are predicted to become an important source of electrical energy in the future. State two advantages of nuclear fusion over nuclear fission.
-
17M.3.sl.TZ1.15a:
State two reagents required to convert vegetable oil to biodiesel.
-
17M.3.sl.TZ1.15b:
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
-
17M.3.sl.TZ1.15c:
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
-
17M.3.sl.TZ1.15d:
Determine the specific energy, in kJg−1, and energy density, in kJcm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 gcm−3; Molar mass = 299 gmol−1;
Enthalpy of combustion = 12.0 MJmol−1.
-
17M.3.sl.TZ1.16a:
State how these gases are produced, giving the appropriate equation(s).
-
17M.3.sl.TZ1.16b:
Outline how the carbon monoxide is then converted to a hydrocarbon fuel.
-
17M.3.sl.TZ1.17a:
Suggest why it is only in recent years that specific predictions of the future effects of fossil fuel combustion have been made.
-
17M.3.sl.TZ1.17b:
Carbon dioxide has two different bond stretching modes illustrated below.

Predict, with an explanation, whether these stretching modes will absorb infrared radiation.
-
17M.3.sl.TZ1.17c:
Outline, giving the appropriate equation(s), how increasing levels of carbon dioxide will affect the pH of the oceans.
-
17M.3.sl.TZ1.17d:
Many combustion processes also release particulate matter into the atmosphere. Suggest, giving your reason, how this might affect the temperature of the Earth’s surface.
-
17M.3.hl.TZ1.18b.ii:
The mass of X is 8.005305 amu and that of is 4.002603 amu. Determine the energy produced, in J, when one atom of is formed in this reaction. Use section 2 of the data booklet.
-
17M.3.hl.TZ1.19a:
Identify two ways in which the structure of the dye shown resembles the chlorophyll molecule. Use section 35 of the data booklet.
-
17M.3.hl.TZ1.19b:
Both photosynthesis and the Grätzel cell use energy from sunlight to bring about reduction. Deduce an equation for the reduction reaction in the electrolyte of a Grätzel cell.
-
17M.3.hl.TZ1.22a:
Deduce half-equations for the reactions at the two electrodes and hence the equation for the overall reaction.
-
17M.3.hl.TZ1.22b.i:
Suggest a way in which they are similar.
-
17M.3.hl.TZ1.22b.ii:
Outline the difference between primary and rechargeable cells.
-
17M.3.hl.TZ1.22c:
Identify one factor that affects the voltage of a cell and a different factor that affects the current it can deliver.
-
17M.3.sl.TZ2.12b:
Coloured molecules absorb sunlight. Identify the bonding characteristics of such molecules.
-
17M.3.sl.TZ2.13a:
State one advantage and one disadvantage for each energy source in the table.
-
17M.3.sl.TZ2.13b.i:
Calculate the specific energy of hydrogen, stating its units. Refer to sections 1, 6 and 13 of the data booklet.
-
17M.3.sl.TZ2.13b.ii:
Hydrogen has a higher specific energy than petrol (gasoline) but is not used as a primary fuel source in cars. Discuss the disadvantages of using hydrogen.
-
17M.3.sl.TZ2.14a:
Identify which region, A or B, corresponds to each type of radiation by completing the table.
-
17M.3.sl.TZ2.14b.i:
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
-
17M.3.sl.TZ2.14b.ii:
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
-
17M.3.sl.TZ2.14c.i:
Suggest an equation for the production of syngas from coal.
-
17M.3.sl.TZ2.14c.ii:
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
-
17M.3.sl.TZ2.14c.iii:
Suggest a reason why syngas may be considered a viable alternative to crude oil.
-
17M.3.sl.TZ2.12a.i:
One fusion reaction occurring in the sun is the fusion of deuterium, , with tritium, , to form helium, . State a nuclear equation for this reaction.
-
17M.3.sl.TZ2.12a.ii:
Explain why this fusion reaction releases energy by using section 36 of the data booklet.
-
17M.3.hl.TZ2.16a.iii:
Calculate the energy released, in MeV, in this reaction, using section 36 of the data booklet.
-
17M.3.hl.TZ2.17c.i:
Deduce the half-cell equations occurring at each electrode during discharge.
-
17M.3.hl.TZ2.17c.ii:
Outline the function of the proton-exchange membrane (PEM) in the fuel cell.
-
17M.3.hl.TZ2.17c.iii:
Explain how the flow of ions allows for the operation of the fuel cell.
-
17M.3.hl.TZ2.18a.ii:
The structures of 11-cis-retinal and β-carotene are given in section 35 of the data booklet. Suggest a possible wavelength of light absorbed by each molecule using section 3 of the data booklet.
-
17M.3.hl.TZ2.19a:
Contrast how absorption of photons and charge separation occur in each device.
-
17M.3.hl.TZ2.19b:
Suggest one advantage a DSSC has over a silicon based photovoltaic cell.
-
17N.3.sl.TZ0.12a:
Calculate the specific energy of octane, C8H18, in kJ kg–1 using sections 1, 6 and 13 of the data booklet.
-
17N.3.sl.TZ0.12b:
A typical wood has a specific energy of 17 × 103 kJ kg–1. Comment on the usefulness of octane and wood for powering a moving vehicle, using your answer to (a).
If you did not work out an answer for (a), use 45 × 103 kJ kg–1 but this is not the correct answer.
-
17N.3.sl.TZ0.12c:
State the name of one renewable source of energy other than wood.
-
17N.3.sl.TZ0.13a:
“Knocking” in an automobile (car) engine can be prevented by increasing the octane number of the fuel. Explain, including an equation with structural formulas, how heptane, C7H16, could be chemically converted to increase its octane number.
-
17N.3.sl.TZ0.13b:
Many like to refer to our “carbon footprint”. Outline one difficulty in quantifying such a concept.
-
17N.3.sl.TZ0.13c:
Climate change or global warming is a consequence of increased levels of carbon dioxide in the atmosphere. Explain how the greenhouse effect warms the surface of the earth.
- 17N.3.sl.TZ0.13d: Outline how water and carbon dioxide absorb infrared radiation.
-
17N.3.sl.TZ0.14a.i:
Compare and contrast fission and fusion in terms of binding energy and the types of nuclei involved.
-
17N.3.sl.TZ0.14a.ii:
Suggest two advantages that fusion has over fission.
-
17N.3.sl.TZ0.14b:
The amount of 228Ac in a sample decreases to one eighth of its original value in about 18 hours due to β-decay. Estimate the half-life of 228Ac.
- 17N.3.sl.TZ0.15a: State the structural feature of chlorophyll that enables it to absorb visible light.
- 17N.3.sl.TZ0.15b: Vegetable oils are too viscous for use as liquid fuels. Describe, using an equation, how a...
-
17N.3.hl.TZ0.18c.i:
Calculate the loss in mass, in kg, and the energy released, in J, when 0.00100 mol of 228Ac decays, each atom losing an electron. Use section 2 of the data booklet and E = mc2.
228Ac → + 228Th
-
17N.3.hl.TZ0.18c.ii:
Determine the energy released, in J, by 0.00100 mol of 228Ac over the course of 18 hours.
- 17N.3.hl.TZ0.18d: Outline how nuclear ionising radiation can damage DNA and enzymes in living cells.
-
17N.3.hl.TZ0.19b:
The natural absorption of light by chlorophyll has been copied by those developing dye-sensitized solar cells (DSSCs). Outline how a DSSC works.
-
17N.3.hl.TZ0.20a:
Deduce the half-equations and the overall equation for the reactions taking place in a direct methanol fuel cell (DMFC) under acidic conditions.
-
17N.3.hl.TZ0.20b:
Outline one advantage and one disadvantage of the methanol cell (DMFC) compared with a hydrogen-oxygen fuel cell.
-
18M.3.hl.TZ1.13a:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
18M.3.hl.TZ1.13b:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.

-
18M.3.hl.TZ1.14a.i:
Complete the half-equations on the diagram and identify the species moving between the electrodes.

-
18M.3.hl.TZ1.14a.ii:
State the factor that limits the maximum current that can be drawn from this cell and how electrodes are designed to maximize the current.
-
18M.3.hl.TZ1.14b.ii:
Explain how the proportion of 235U in natural uranium is increased.
-
18M.3.hl.TZ1.15a:
Early photovoltaic cells were based on silicon containing traces of other elements. State the type of semiconductor produced by doping silicon with indium, In, giving a reason that refers to its electronic structure.
-
18M.3.hl.TZ1.15b:
Dye-sensitized solar cells, DSSCs, use a dye to absorb the sunlight. State two advantages that DSSCs have over traditional silicon based photovoltaic cells.
-
18M.3.hl.TZ1.15c:
The structure of two dyes used in DSSCs are shown.

Predict, giving a reason, which dye will absorb light of longer wavelength.
-
18M.3.hl.TZ2.13c:
Fuel cells have a higher thermodynamic efficiency than octane. The following table gives some information on a direct methanol fuel cell.

Determine the thermodynamic efficiency of a methanol fuel cell operating at 0.576 V.
Use sections 1 and 2 of the data booklet.
-
18M.3.hl.TZ2.16c.i:
Calculate the relative rate of effusion of 235UF6(g) to 238UF6(g) using sections 1 and 6 of the data booklet.
-
18M.3.hl.TZ2.16c.ii:
Explain, based on molecular structure and bonding, why diffusion or centrifuging can be used for enrichment of UF6 but not UO2.
-
18M.3.hl.TZ2.18a:
Draw the Lewis (electron dot) structure for an appropriate doping element in the box in the centre identifying the type of semiconductor formed.

-
18M.3.hl.TZ2.18b.i:
State the feature of the molecules responsible for the absorption of light.
-
18M.3.hl.TZ2.18b.ii:
Outline why complex B absorbs light of longer wavelength than complex A.
-
18M.3.sl.TZ1.9a:
Identify one naturally occurring greenhouse gas, other than carbon dioxide or water vapour, and its natural source.
-
18M.3.sl.TZ1.9b:
Formulate an equation that shows how aqueous carbon dioxide produces hydrogen ions, H+(aq).
-
18M.3.sl.TZ1.9c:
The concentrations of oxygen and nitrogen in the atmosphere are much greater than those of greenhouse gases. Outline why these gases do not absorb infrared radiation.
-
18M.3.sl.TZ1.10a:
Identify an element, other than carbon and hydrogen, found at significant concentrations in fossil fuels.
-
18M.3.sl.TZ1.10b:
Petroleum contains many hydrocarbons. Explain how these are separated by fractional distillation.
-
18M.3.sl.TZ1.10c.i:
Determine the specific energy and energy density of petrol (gasoline), using data from sections 1 and 13 of the data booklet. Assume petrol is pure octane, C8H18. Octane: molar mass = 114.26 g mol−1, density = 0.703 g cm−3.
-
18M.3.sl.TZ1.10c.ii:
Outline why the energy available from an engine will be less than these theoretical values.
-
18M.3.sl.TZ1.11a.i:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
18M.3.sl.TZ1.11a.ii:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
-
18M.3.sl.TZ1.11b:
Outline why biofuels are considered more environmentally friendly, even though they produce more carbon dioxide per kJ of energy than petroleum based fuels.
-
18M.3.sl.TZ1.12a.ii:
Explain how 235U fission results in a chain reaction, including the concept of critical mass.
-
18M.3.sl.TZ1.12b:
Suggest one reason why there is opposition to the increased use of nuclear fission reactors.
-
18M.3.sl.TZ2.10a:
Outline two reasons why oil is one of the world’s significant energy sources.
-
18M.3.sl.TZ2.10b.i:
Formulate an equation for the cracking of C16H34 into two products with eight carbon atoms each.
-
18M.3.sl.TZ2.10b.ii:
Identify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
-
18M.3.sl.TZ2.10c.i:
Outline how higher octane fuels help eliminate “knocking” in engines.
-
18M.3.sl.TZ2.10c.ii:
The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
-
18M.3.sl.TZ2.11a:
Explain the molecular mechanism by which carbon dioxide acts as a greenhouse gas.
-
18M.3.sl.TZ2.11b:
Discuss the significance of two greenhouse gases, other than carbon dioxide, in causing global warming or climate change.
-
18M.3.sl.TZ2.12a:
Calculate the thermal efficiency of a steam turbine supplied with steam at 540°C and using a river as the choice of sink at 23 °C.
-
18M.3.sl.TZ2.12b:
Power plants generating electricity by burning coal to boil water operate at approximately 35% efficiency.
State what this means and suggest why it is lower than the thermal efficiency.
-
18M.3.sl.TZ2.13a:
Compare and contrast the process of nuclear fusion with nuclear fission.
-
18M.3.sl.TZ2.13b:
Dubnium-261 has a half-life of 27 seconds and rutherfordium-261 has a half-life of 81 seconds.
Estimate what fraction of the dubnium-261 isotope remains in the same amount of time that of rutherfordium-261 decays.
-
18M.3.sl.TZ2.14a:
Deduce the equation for the transesterification reaction of pentyl octanoate, C7H15COOC5H11, with methanol.
-
18M.3.sl.TZ2.14b:
Outline why the ester product of this reaction is a better diesel fuel than pentyl octanoate.
- 18N.3.sl.TZ0.9a: Explain fusion reactions with reference to binding energy.
- 18N.3.sl.TZ0.9b.i: Outline why the term breeder is used for the reactors.
- 18N.3.sl.TZ0.9b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
-
18N.3.sl.TZ0.9c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
-
18N.3.sl.TZ0.10a:
Formulate equation(s) for the conversion of coal and steam to methane.
-
18N.3.sl.TZ0.10b.i:
Calculate the specific energy, in kJ g−1, of methane.
-
18N.3.sl.TZ0.10b.ii:
Comment on the specific energies of hydrogen and methane.
-
18N.3.sl.TZ0.10c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
- 18N.3.sl.TZ0.11a: Suggest another advantage and one disadvantage of solar energy.
- 18N.3.sl.TZ0.11b: Light can be absorbed by chlorophyll and other pigments. Consider molecules A and B represented...
- 18N.3.sl.TZ0.11c.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
- 18N.3.sl.TZ0.11c.ii: Describe how vegetable oils can be converted to a more suitable fuel.
- 18N.3.sl.TZ0.11d: Contrast the importance of carbon dioxide and methane as greenhouse gases.
-
18N.3.sl.TZ0.11e:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
- 18N.3.hl.TZ0.12a: Explain fusion reactions with reference to binding energy.
- 18N.3.hl.TZ0.12b.i: Outline why the term breeder is used for the reactors.
- 18N.3.hl.TZ0.12b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
-
18N.3.hl.TZ0.12d.i:
Deduce a Lewis (electron dot) structure of the superoxide, O2–, free radical.
- 18N.3.hl.TZ0.12d.ii: Explain why free radicals are harmful to living cells.
-
18N.3.hl.TZ0.12c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
-
18N.3.hl.TZ0.13a:
Formulate equation(s) for the conversion of coal and steam to methane.
-
18N.3.hl.TZ0.13b:
Comment on the specific energies of hydrogen and methane.
-
18N.3.hl.TZ0.13c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
- 18N.3.hl.TZ0.14a: Suggest another advantage and one disadvantage of solar energy.
- 18N.3.hl.TZ0.14b.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
- 18N.3.hl.TZ0.14b.ii: Describe how vegetable oils can be converted to a more suitable fuel.
- 18N.3.hl.TZ0.14c: Contrast the importance of carbon dioxide and methane as greenhouse gases.
-
18N.3.hl.TZ0.14d:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
- 18N.3.hl.TZ0.15a: Outline how a rechargeable battery differs from a primary cell.
-
18N.3.hl.TZ0.15b:
Formulate half-equations for the reactions at the anode (negative electrode) and cathode (positive electrode) during discharge of a lithium-ion battery.
-
18N.3.hl.TZ0.15c:
A voltaic cell consists of a nickel electrode in 1.0 mol dm−3 Ni2+ (aq) solution and a cadmium electrode in a Cd2+ (aq) solution of unknown concentration.
Cd (s) + Ni2+ (aq) → Cd2+ (aq) + Ni (s) EΘcell = 0.14 V
Determine the concentration of the Cd2+ (aq) solution if the cell voltage, E, is 0.19 V at 298 K. Use section 1 of the data booklet.
- 18N.3.hl.TZ0.15d.i: Identify the structural feature of the dye that allows the conversion of solar energy into...
- 18N.3.hl.TZ0.15d.ii: Outline the effect of sunlight on the dye in the solar cell.
- 18N.3.hl.TZ0.15d.iii: State the purpose of TiO2.
-
18N.3.hl.TZ0.15d.iv:
Deduce the reduction half-equation at the cathode.
-
19M.3.hl.TZ1.15a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.hl.TZ1.15b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.hl.TZ1.15b(ii):
Hydroelectric power plants produced 16% of the world’s energy in 2015, down from 21% in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.hl.TZ1.15c(i):
Methane can also be obtained by fractional distillation of crude oil.
[Source: Image used with kind permission of science-resources.co.uk]
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.hl.TZ1.15c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.hl.TZ1.15d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.hl.TZ1.15d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.hl.TZ1.16a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.hl.TZ1.16a(ii):
Outline why the reaction releases energy.
-
19M.3.hl.TZ1.16a(iii):
The masses of the particles involved in this fission reaction are shown below.
Mass of neutron = 1.00867 amu
Mass of U-235 nucleus = 234.99346 amu
Mass of Ba-144 nucleus = 143.89223 amu
Mass of Kr-89 nucleus = 88.89788 amuDetermine the energy released, in J, when one uranium-235 nucleus undergoes fission. Use this data and information from sections 1 and 2 of the data booklet.
-
19M.3.hl.TZ1.16b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.hl.TZ1.16c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for its radioactivity to fall to 10% of its initial value, using section 1 of the data booklet.
-
19M.3.hl.TZ1.17a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.hl.TZ1.17b(i):
Ethanol can be used in a direct-ethanol fuel cell (DEFC) as illustrated by the flow chart.
Deduce the half-equations occurring at electrodes A and B.
Electrode A:
Electrode B:
-
19M.3.hl.TZ1.17b(ii):
State the name and function of X in the diagram in (b)(i).
Name:
Function:
-
19M.3.hl.TZ1.17b(iii):
Outline why aqueous ethanol, rather than pure ethanol, is used in a DEFC.
-
19M.3.hl.TZ1.17c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.hl.TZ1.18a:
Some solar cells use photovoltaic semi-conductors. Compare, giving reasons, the electrical conductivity of metals and semi-conductors as temperature increases.
-
19M.3.hl.TZ1.18b:
Suggest one advantage of a dye-sensitized solar cell (DSSC) over a silicon based photovoltaic cell.
-
19M.3.hl.TZ2.14:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.hl.TZ2.15a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.hl.TZ2.15b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19M.3.hl.TZ2.15c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
-
19M.3.hl.TZ2.16a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.hl.TZ2.16a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.hl.TZ2.16a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.hl.TZ2.16b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
-
19M.3.hl.TZ2.16c:
Outline how the energy of a fission reaction can be calculated.
-
19M.3.hl.TZ2.16d:
Calculate the half-life of an isotope whose mass falls from 5.0 × 10−5 g to 4.0 × 10−5 g in 31.4 s, using section 1 of the data booklet.
-
19M.3.hl.TZ2.17:
This question is about biofuel.
Evaluate the use of biodiesel in place of diesel from crude oil.
-
19M.3.hl.TZ2.18a:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.hl.TZ2.18b:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.hl.TZ2.19a:
Outline how a microbial fuel cell produces an electric current from glucose.
C6H12O6 (aq) + 6O2 (g) → 6CO2 (g) + 6H2O (l)
-
19M.3.hl.TZ2.19b:
The cell potential for the spontaneous reaction when standard magnesium and silver half-cells are connected is +3.17 V.
Determine the cell potential at 298 K when:
[Mg2+] = 0.0500 mol dm−3
[Ag+] = 0.100 mol dm−3Use sections 1 and 2 of the data booklet.
- 19M.3.hl.TZ2.19c: Outline one difference between a primary and a secondary cell.
-
19M.3.hl.TZ2.20a:
Sketch graphs to show the general effect of increasing temperature on the electrical conductivity of semiconductors and metals on the axes below.
-
19M.3.hl.TZ2.20b:
Explain the function of dyes in a dye-sensitized solar cell (DSSC).
-
19M.3.sl.TZ1.11a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.sl.TZ1.11b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.sl.TZ1.11b(ii):
Hydroelectric power plants produced 16 % of the world’s energy in 2015, down from 21 % in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.sl.TZ1.11c(i):
Methane can also be obtained by fractional distillation of crude oil.
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.sl.TZ1.11c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.sl.TZ1.11d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.sl.TZ1.11d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.sl.TZ1.12a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.sl.TZ1.12a(ii):
Outline why the reaction releases energy.
-
19M.3.sl.TZ1.12b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.sl.TZ1.12c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for the mass of 89Kr to fall to 6.25 % of its initial value.
-
19M.3.sl.TZ1.13a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.sl.TZ1.13b:
Show that, for combustion of equal masses of fuel, ethanol (Mr = 46 g mol−1) has a lower carbon footprint than octane (Mr = 114 g mol−1).
-
19M.3.sl.TZ1.13c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.sl.TZ2.9:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.sl.TZ2.10a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.sl.TZ2.10b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19M.3.sl.TZ2.10c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
-
19M.3.sl.TZ2.11a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.sl.TZ2.11a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.sl.TZ2.11a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.sl.TZ2.11b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
-
19M.3.sl.TZ2.11c:
90Sr, a common product of fission, has a half-life of 28.8 years.
Determine the number of years for the activity of a sample of 90Sr to fall to one eighth () of its initial value.
-
19M.3.sl.TZ2.12a:
The structure of chlorophyll is given in section 35 of the data booklet.
State the feature of the chlorophyll molecule that enables it to absorb light in the visible spectrum.
-
19M.3.sl.TZ2.12b:
Evaluate the use of biodiesel in place of diesel from crude oil.
Strength:
Limitation:
-
19M.3.sl.TZ2.13a:
State one greenhouse gas, other than carbon dioxide.
-
19M.3.sl.TZ2.13b:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.sl.TZ2.13c:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19N.3.sl.TZ0.11a:
Discuss the data.
- 19N.3.hl.TZ0.16b: Outline what is meant by the degradation of energy.
- 19N.3.sl.TZ0.12a: Suggest why a high-octane number fuel is preferable.
- 19N.3.sl.TZ0.12b(i): Reforming reactions are used to increase the octane number of a hydrocarbon fuel. Suggest the...
- 19N.3.sl.TZ0.12b(ii): The 1H NMR spectrum of one of the products has four signals. The integration trace shows a ratio...
-
19N.3.sl.TZ0.13a(i):
State the nuclear equation for the fusion reaction.
- 19N.3.sl.TZ0.13a(ii): Explain why fusion is an exothermic process.
-
19N.3.hl.TZ0.18a(iii):
Calculate the heat energy released, in J, by the fusion reaction producing one atom of carbon-12. Use section 2 of the data booklet and E = mc2.
-
19N.3.sl.TZ0.13b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
-
19N.3.sl.TZ0.14a:
Write the equation for the complete combustion of ethanol.
-
19N.3.sl.TZ0.14b:
Outline the evidence that relates global warming to increasing concentrations of greenhouse gases in the atmosphere.
-
19N.3.sl.TZ0.14c:
Explain, including a suitable equation, why biofuels are considered to be carbon neutral.
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
-
19N.3.hl.TZ0.20b(i):
Calculate the cell potential, Eθ, in V, using section 24 of the data booklet.
-
19N.3.hl.TZ0.20b(ii):
Suggest how PEM fuel cells can be used to produce a larger voltage than that calculated in (b)(i).
- 19N.3.hl.TZ0.20c: Suggest an advantage of the PEM fuel cell over the lead-acid battery for use in cars.
- 19N.3.hl.TZ0.20d(i): Outline the functions of the dye, TiO2 and the electrolyte in the operation of the...
- 19N.3.hl.TZ0.20d(ii): Suggest an advantage of the DSSC over silicon-based photovoltaic cells.
-
19N.3.sl.TZ0.11b:
In a natural gas power station, 1.00 tonne of natural gas produces 2.41 × 104 MJ of electricity.
Calculate the percentage efficiency of the power station.
1 tonne = 1000 kg
Specific energy of natural gas used = 55.4 MJ kg−1 - 19N.3.sl.TZ0.14d: State the type of reaction that occurs when ethanol reacts with vegetable oil to form biodiesel.
-
19N.3.hl.TZ0.16a:
Discuss the data.
-
19N.3.hl.TZ0.18a(i):
State the nuclear equation for the fusion reaction.
- 19N.3.hl.TZ0.18a(ii): Explain why fusion is an exothermic process.
-
19N.3.hl.TZ0.18b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
-
20N.3.sl.TZ0.9a:
Calculate the energy released, in , from the complete combustion of of ethanol.
- 20N.3.sl.TZ0.9b: State a class of organic compounds found in gasoline.
-
20N.3.sl.TZ0.9c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
-
20N.3.sl.TZ0.9d:
A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
- 20N.3.sl.TZ0.9e: Contrast the molecular structures of biodiesel and the vegetable oil from which it is formed.
-
20N.3.sl.TZ0.9f(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
-
20N.3.sl.TZ0.9f(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
-
20N.3.sl.TZ0.10b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
- 20N.3.sl.TZ0.10c: Outline a health risk produced by exposure to radioactive decay.
-
20N.3.sl.TZ0.10d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
- 20N.3.sl.TZ0.10e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
-
20N.3.hl.TZ0.11a:
Calculate the energy released, in , from the complete combustion of of ethanol.
- 20N.3.hl.TZ0.11b: State a class of organic compounds found in gasoline.
-
20N.3.hl.TZ0.11c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
- 20N.3.hl.TZ0.11d: A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture...
-
20N.3.hl.TZ0.11e(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
-
20N.3.hl.TZ0.11e(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
-
20N.3.hl.TZ0.11e(iv):
Determine the relative rate of effusion of methane () to carbon dioxide (), under the same conditions of temperature and pressure. Use section 1 of the data booklet.
-
20N.3.hl.TZ0.12b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
- 20N.3.hl.TZ0.12c: Outline a health risk produced by exposure to radioactive decay.
-
20N.3.hl.TZ0.12d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
- 20N.3.hl.TZ0.12e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
-
20N.3.hl.TZ0.12f:
Determine the nuclear binding energy, in , of using sections 2 and 4 of the data booklet.
The mass of the nucleus is .
-
20N.3.hl.TZ0.14a:
Doping of silicon increases the conductivity in semiconductors.
Describe the doping in p-type and n-type semiconductors.
- 20N.3.hl.TZ0.14b: Doping of silicon increases the conductivity in semiconductors. Explain how doping improves the...
D: Medicinal chemistry
-
16N.3.sl.TZ0.16a:
(i) Outline what is meant by the term “ring strain”.
(ii) On the diagram above, label with asterisk/s (*) the carbon atom/s that experience ring strain.
-
16N.3.sl.TZ0.16b:
(i) Some antibiotic-resistant bacteria produce a beta-lactamase enzyme which destroys penicillin activity. Suggest how adding clavulanic acid to penicillin enables the antibiotic to retain its activity.
(ii) Populations of antibiotic-resistant bacteria have increased significantly over the last 60 years. Outline why antibiotics such as penicillin should not be prescribed to people suffering from a viral infection.
- 16N.3.sl.TZ0.17a: Zanamivir must be taken by inhalation, not orally. Deduce what this suggests about the...
- 16N.3.sl.TZ0.17c: The synthesis of oseltamivir is dependent on a supply of the precursor shikimic acid, which is...
- 16N.3.sl.TZ0.18d: State why aspirin is described as a mild analgesic with reference to its site of action.
-
16N.3.sl.TZ0.19a:
Ranitidine (Zantac) is a drug that inhibits stomach acid production. Outline why the development of this drug was based on a detailed knowledge of the structure of histamine, shown below.
-
16N.3.sl.TZ0.19b:
Two other drugs, omeprazole (Prilosec) and esomeprazole (Nexium), directly prevent the release of acid into the stomach. Identify the site of action in the body.
-
16N.3.sl.TZ0.19c:
A different approach to treating excess stomach acid is to neutralize it with antacids. Formulate an equation that shows the action of an antacid that can neutralize three moles of hydrogen ions, H+, per mole of antacid.
-
16N.3.sl.TZ0.20b:
Methadone is sometimes used to help reduce withdrawal symptoms in the treatment of heroin addiction. Outline one withdrawal symptom that an addict may experience.
- 16N.3.hl.TZ0.26c: Omeprazole exists as a racemic mixture whereas esomeprazole is a single enantiomer. Outline how,...
-
16N.3.hl.TZ0.28a:
Deduce equations for the following nuclear reactions:
(i) Molybdenum-98 absorbs a neutron.
(ii) The isotope produced in (a) (i) decays into technetium-99m.
- 16N.3.hl.TZ0.28b: Molybdenum-99 has a half-life of 66 hours, while technetium-99m has a half-life of 6 hours....
- 16N.3.hl.TZ0.28c: Outline two reasons, other than its half-life, why technetium-99m is so useful in medical diagnosis.
- 16N.3.hl.TZ0.28d: Outline the nature of the radioactive waste that is generated by the use of technetium-99m in...
- 16N.3.hl.TZ0.29a: Suggest what may have led to these changes in acceptable concentrations.
-
16N.3.hl.TZ0.29b:
One class of performance-enhancing drugs is the anabolic steroids. Detection of these drugs in urine samples uses a combination of gas chromatography and mass spectrometry (GC/MS).
(i) Describe how gas chromatography enables the components of urine to be analysed.
(ii) The structures of two steroids, testosterone and nandrolone, are given below.
With reference to the molar masses of the two steroids, determine, with a reason, which can be identified from the mass spectrum below.
-
17M.3.sl.TZ1.18a:
Dose response curves are determined for each drug.

Outline the significance of range “a”.
-
17M.3.sl.TZ1.18b.i:
Suggest the type of reaction used to convert morphine to codeine.
-
17M.3.sl.TZ1.18b.ii:
State and explain the action of opiates as painkillers.
-
17M.3.sl.TZ1.19a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.sl.TZ1.19b:
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
-
17M.3.sl.TZ1.19c:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
17M.3.sl.TZ1.19d:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
17M.3.sl.TZ1.19e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
17M.3.sl.TZ1.20a:
Calculate the pH of the buffer from the following data and section 1 of the data booklet.
pKa(CO2) = 6.34
[HCO3−(aq)] = 1.40 × 10−2 moldm−3
[CO2(aq)] = 1.25 × 10−3 moldm−3
-
17M.3.sl.TZ1.20b:
Explain the effect of a large amount of aspirin on the pH of blood.
-
17M.3.sl.TZ1.21a:
Outline how oseltamivir (Tamiflu®) works.
-
17M.3.sl.TZ1.21b:
Oseltamivir was commercially produced from shikimic acid, a precursor which is a metabolite in micro-organisms and plants.
Outline how green chemistry was used to develop the precursor for oseltamivir in order to overcome a shortage of the drug during the flu season.
-
17M.3.sl.TZ1.21c:
Suggest why the administration of antibiotics to humans and animals can affect the environment.
-
17M.3.hl.TZ1.25d:
Some mild analgesics contain a solid mixture of acidic aspirin and a non-acidic organic chemical of similar polarity to asprin.
Discuss how acid-base properties and the process of solvent extraction can be used to separate aspirin from the mixture.
-
17M.3.hl.TZ1.28b:
A mixture of enantiomers shows optical rotation.
Suggest a conclusion you can draw from this data.
-
17M.3.hl.TZ1.29a:
Yttrium-90 is used in treating certain cancers.
Formulate a nuclear equation for the beta decay of yttrium-90.
-
17M.3.hl.TZ1.29b:
Lutetium-177 is a common isotope used for internal radiation therapy.
Suggest why lutetium-177 is an ideal isotope for the treatment of certain cancers based on the type of radiation emitted.
-
17M.3.hl.TZ1.29c.i:
Calculate the rate constant, , in day−1, for the decay of iodine-131 using section 1 of the data booklet.
-
17M.3.hl.TZ1.29c.ii:
Calculate the time, in days, for 90% of the sample to decay.
-
17M.3.hl.TZ1.29d:
A breathalyser measures the blood alcohol content from a breath sample. Formulate half-equations for the reactions at the anode (negative electrode) and the cathode (positive electrode) in a fuel cell breathalyser.
-
17M.3.sl.TZ2.15a.iii:
State two techniques which could be used to confirm the identity of aspirin.
-
17M.3.sl.TZ2.15b.i:
State how aspirin can be converted to water-soluble aspirin.
-
17M.3.sl.TZ2.15b.ii:
Compare, giving a reason, the bioavailability of soluble aspirin with aspirin.
-
17M.3.sl.TZ2.16a:
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
-
17M.3.sl.TZ2.16b:
Suggest a reagent used to prepare diamorphine from morphine.
-
17M.3.sl.TZ2.16c:
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
-
17M.3.sl.TZ2.17a:
Two drugs are ranitidine (Zantac) and omeprazole (Prilosec). Outline how they function to reduce stomach acidity.
-
17M.3.sl.TZ2.17b:
0.500 g of solid anhydrous sodium carbonate, Na2CO3(s), is dissolved in 75.0 cm3 of 0.100 moldm−3 sodium hydrogen carbonate solution, NaHCO3(aq). Assume the volume does not change when the salt dissolves.
HCO3−(aq) CO32−(aq) + H+(aq) pKa = 10.35.
Calculate the pH of the buffer solution.
-
17M.3.sl.TZ2.18a.i:
Compare and contrast the structures of oseltamivir and zanamivir, stating the names of functional groups.
-
17M.3.sl.TZ2.18a.ii:
Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds, using section 26 of the data booklet.
-
17M.3.sl.TZ2.18b:
Suggest one ethical consideration faced by medical researchers when developing medications.
-
17M.3.sl.TZ2.19a:
Suggest one problem associated with chlorinated organic solvents as chemical waste.
-
17M.3.sl.TZ2.19b:
Suggest how the principles of green chemistry can be used to solve the environmental problems caused by organic solvents.
-
17M.3.hl.TZ2.20a.iv:
State two techniques, other than IR spectroscopy, which could be used to confirm the identity of aspirin.
-
17M.3.hl.TZ2.22a:
Outline how ranitidine (Zantac) functions to reduce stomach acidity.
-
17M.3.hl.TZ2.25:
Taxol is produced using a chiral auxiliary. Describe how the chiral auxiliary functions to produce the desired product.
-
17M.3.hl.TZ2.26a.i:
Explain why alpha-radiation is particularly suitable for this treatment.
-
17M.3.hl.TZ2.26a.ii:
Outline how the alpha-radiation in TAT is directed to cancer cells.
-
17M.3.hl.TZ2.26b.i:
Identify the type of radiation emitted by these two radioisotopes.
-
17M.3.hl.TZ2.26b.ii:
State an equation for the one-step decay of yttrium-90.
-
17M.3.hl.TZ2.26b.iii:
The half-life of lutetium-177 is 6.75 days. Calculate the percentage remaining after 27 days.
-
17N.3.sl.TZ0.16:
Radioisotopes are used to diagnose and treat various diseases. Explain the low environmental impact of most medical nuclear waste.
- 17N.3.sl.TZ0.17a: Aspirin is a mild analgesic derived from salicylic acid found in willow bark. Describe how mild...
-
17N.3.sl.TZ0.17b.i:
The strong analgesics morphine and codeine are opiates. Outline how codeine can be synthesized from morphine. The structures of morphine and codeine are in section 37 of the data booklet.
-
17N.3.sl.TZ0.17b.ii:
Explain why opiates are addictive.
-
17N.3.sl.TZ0.18a:
Outline the difference between the therapeutic index in animal studies and the therapeutic index in humans.
- 17N.3.sl.TZ0.18b: State the method of drug administration that gives the maximum bioavailability.
-
17N.3.sl.TZ0.19a:
State the names of two functional groups that both compounds contain, using section 37 of the data booklet.
- 17N.3.sl.TZ0.19b: Explain how oseltamivir and zanamivir can stop the spread of the flu virus in the body.
-
17N.3.sl.TZ0.20a:
Explain how ranitidine (Zantac) reduces stomach acid production.
-
17N.3.sl.TZ0.20b:
The pH is maintained in different fluids in the body by the use of buffers.
Calculate the pH of a buffer solution of 0.0200 mol dm–3 carbonic acid, H2CO3, and 0.400 mol dm–3 sodium hydrogen carbonate, NaHCO3. The pKa of carbonic acid is 6.35.
- 17N.3.sl.TZ0.21: Molecules of antibiotics often contain a beta-lactam ring. Explain the importance of the...
-
17N.3.hl.TZ0.21a:
State a nuclear equation to show the decay of lutetium-177.
-
17N.3.hl.TZ0.21b:
The half-life of lutetium-177 is 6.73 days. Determine the percentage of a sample of lutetium-177 remaining after 14.0 days.
-
17N.3.hl.TZ0.21c:
Explain the low environmental impact of most medical nuclear waste.
- 17N.3.hl.TZ0.22a.i: Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
-
17N.3.hl.TZ0.22a.ii:
Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section 26 of the data booklet.
- 17N.3.hl.TZ0.22b: Describe how mild analgesics function.
-
17N.3.hl.TZ0.23b:
Explain the role of the chiral auxiliary in the synthesis of Taxol.
- 17N.3.hl.TZ0.27: Ethanol slows down the reaction time of a driver leading to traffic accidents. Explain how the...
-
18M.3.hl.TZ1.16e:
Many drugs are chiral. Explain how a polarimeter can be used to determine the relative proportion of two enantiomers.
-
18M.3.hl.TZ1.19a:
Describe how ionizing radiation destroys cancer cells.
-
18M.3.hl.TZ1.19b:
Outline how Targeted Alpha Therapy (TAT) is used for treating cancers that have spread throughout the body.
-
18M.3.hl.TZ1.20a:
Hexane and propanone have vapour pressures of 17 kPa and 24 kPa respectively at 20 °C.
Calculate the vapour pressure, in kPa, at 20 °C of a mixture containing 60% hexane and 40% propanone by mole fraction, using Raoult’s law and assuming the mixture is ideal.
-
18M.3.hl.TZ1.20b:
Explain how hexane and propanone may be separated by fractional distillation.
-
18M.3.hl.TZ2.25:
Taxol was originally obtained from the bark of the Pacific yew tree.
Outline how Green Chemistry has improved the process of obtaining Taxol.
-
18M.3.hl.TZ2.26a:
Phosphorous-32 undergoes beta decay. Formulate a balanced nuclear equation for this process.
-
18M.3.hl.TZ2.26b:
The half-life of phosphorus-32 is 14.3 days. Calculate the mass, in g, of 32P remaining after 57.2 days if the initial sample contains 2.63 × 10−8 mol. Use table 1 of the data booklet and Mr = 31.97 g mol−1.
-
18M.3.hl.TZ2.26c:
Explain the targeted alpha therapy (TAT) technique and why it is useful.
-
18M.3.hl.TZ2.27a:
Fuel cells use an electrochemical process to determine the concentration of ethanol.
Formulate the overall equation for this process.
-
18M.3.hl.TZ2.27b:
Predict the chemical shifts and integration for each signal in the 1H NMR spectrum for ethanol using section 27 of the data booklet.
-
18M.3.sl.TZ1.13a:
Aspirin is often taken to reduce pain, swelling or fever. State one other use of aspirin.
-
18M.3.sl.TZ1.13b.i:
State what is meant by the bioavailability of a drug.
-
18M.3.sl.TZ1.13b.ii:
Outline how the bioavailability of aspirin may be increased.
-
18M.3.sl.TZ1.13c.i:
Compare and contrast the IR spectrum of aspirin with that of salicylic acid, using section 26 of the data booklet.
-
18M.3.sl.TZ1.13c.ii:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ1.13c.iii:
Outline two consequences of prescribing antibiotics such as penicillin unnecessarily.
-
18M.3.sl.TZ1.13c.iv:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ1.13d.i:
Morphine and codeine are strong analgesics. Outline how strong analgesics function.
-
18M.3.sl.TZ1.13d.ii:
Suggest one reason why codeine is more widely used than morphine as an analgesic.
-
18M.3.sl.TZ1.14a.i:
An antacid tablet contains 680 mg of calcium carbonate, CaCO3, and 80 mg of magnesium carbonate, MgCO3.
State the equation for the reaction of magnesium carbonate with hydrochloric acid.
-
18M.3.sl.TZ1.14a.ii:
Determine the amount, in mol, of hydrochloric acid neutralized by one antacid tablet.
-
18M.3.sl.TZ1.14b:
Explain how omeprazole (Prilosec) reduces stomach acidity.
-
18M.3.sl.TZ1.15a:
Oseltamivir (Tamiflu) and zanamivir (Relenza) are used against flu viruses. Explain how these drugs function.
-
18M.3.sl.TZ1.15b:
Shikimic acid, the precursor for oseltamivir (Tamiflu), was originally extracted from star anise, and is now produced using genetically modified E. coli bacteria.
Suggest one difficulty associated with synthesizing oseltamivir (Tamiflu) from star anise.
-
18M.3.sl.TZ2.15:
Drug testing is necessary to determine safe and effective doses.
Distinguish between the lethal dose (LD50) and the toxic dose (TD50).
-
18M.3.sl.TZ2.16a.i:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ2.16a.ii:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ2.16b:
State the type of reaction used to synthesize aspirin from salicylic acid.
-
18M.3.sl.TZ2.16c:
Explain why aspirin is not stored in a hot, humid location.
-
18M.3.sl.TZ2.17:
Morphine and diamorphine (heroin) are both opioids.
Explain why diamorphine is more potent than morphine using section 37 of the data booklet.
-
18M.3.sl.TZ2.18a:
Formulate a chemical equation for the neutralization of stomach acid with calcium carbonate.
-
18M.3.sl.TZ2.18b:
Calculate the amount, in mol, of stomach acid neutralized by an antacid tablet containing 0.750 g calcium carbonate.
-
18M.3.sl.TZ2.18c:
Explain how omeprazole (Prilosec) regulates pH in the stomach.
-
18M.3.sl.TZ2.19a:
Identify the names of two functional groups present in zanamivir using section 37 of the data booklet.
-
18M.3.sl.TZ2.19b:
Distinguish between bacteria and viruses.
-
18M.3.sl.TZ2.20:
Drug synthesis often involves solvents.
Identify a common hazardous solvent and a Green solvent that could replace it.
- 18N.3.sl.TZ0.12a: State the internal bond angles in the β-lactam ring and the expected bond angles for the same...
- 18N.3.sl.TZ0.12b: Explain how the open β-lactam ring kills bacteria.
- 18N.3.sl.TZ0.12c: Outline one effect of over-prescription of penicillin.
- 18N.3.sl.TZ0.12d: State how the structure of penicillin can be changed to combat this effect.
- 18N.3.sl.TZ0.12e: Suggest why human cells are not affected by penicillin.
-
18N.3.sl.TZ0.13a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
- 18N.3.sl.TZ0.13b: Describe the analgesic action of an opiate.
- 18N.3.sl.TZ0.13c: Outline the meaning of the bioavailability of a drug.
-
18N.3.sl.TZ0.14a:
Determine the pH of a buffer solution that is 0.0100 mol dm−3 sodium hydrogen carbonate and 0.0200 mol dm−3 sodium carbonate, using section 1 of the data booklet.
Ka (hydrogen carbonate ion) = 4.8 × 10−11
-
18N.3.sl.TZ0.14b:
State the equation for the reaction of calcium carbonate, the active ingredient in some antacids, with stomach acid.
- 18N.3.sl.TZ0.14c: Suggest a technique for measuring the percentage mass of calcium carbonate in this type of...
-
18N.3.sl.TZ0.15a:
State one way in which viruses differ from bacteria.
- 18N.3.sl.TZ0.15b: Outline two different ways in which antiviral medications work.
-
18N.3.sl.TZ0.16:
Suggest two reasons why chlorinated solvents should neither be released into the atmosphere nor incinerated (burnt).
- 18N.3.hl.TZ0.16a: State the internal bond angles in the b-lactam ring and the expected bond angles in sp2 and sp3...
- 18N.3.hl.TZ0.16b: Explain how the open β-lactam ring kills bacteria.
- 18N.3.hl.TZ0.16c: State how the structure of penicillin can be modified to combat the effect of resistance caused...
- 18N.3.hl.TZ0.16d: Suggest why human cells are not affected by penicillin.
-
18N.3.hl.TZ0.17a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
- 18N.3.hl.TZ0.17b: Outline the meaning of the bioavailability of a drug.
- 18N.3.hl.TZ0.19: Outline two different ways in which antiviral medications work.
-
18N.3.hl.TZ0.21a:
The diagram shows part of a Taxol molecule in skeletal form.
Draw a circle around each chiral carbon.
- 18N.3.hl.TZ0.21b: Outline how chiral auxiliaries are used to synthesize the desired enantiomer.
- 18N.3.hl.TZ0.21c: Explain the process of solvent extraction by which Taxol is isolated.
-
18N.3.hl.TZ0.22a:
Alpha particles are more damaging to human cells than any other nuclear radiation and yet they are used in targeted alpha therapy (TAT).
Explain how TAT is relatively safe to use in the treatment of dispersed cancers.
-
18N.3.hl.TZ0.22b.i:
Technetium-99m () has a half-life of 6.0 hours. Calculate the percentage of remaining in a sample of the radioisotope after two days.
-
18N.3.hl.TZ0.22b.ii:
Suggest why the percentage of technetium-99m remaining in the human body two days after injection will be lower than that calculated in (b)(i).
-
18N.3.hl.TZ0.23a:
State an analytical technique used to separate anabolic steroids from other compounds in an athlete’s urine or blood.
-
18N.3.hl.TZ0.23b:
Ethanol in breath can be detected by a redox reaction. Outline this method of detection. An equation is not required.
-
17M.3.hl.TZ1.25a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.hl.TZ1.25b:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
17M.3.hl.TZ1.25c:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
17M.3.hl.TZ1.25e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
19M.3.hl.TZ1.19a:
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that diff erentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.hl.TZ1.20a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.hl.TZ1.20b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.hl.TZ1.20b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.hl.TZ1.21a:
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.hl.TZ1.21b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.hl.TZ1.22a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.hl.TZ1.22a(ii):
The resulting active metabolite of oseltamivir can be detected by mass spectrometry (MS) analysis.
Deduce the mass of the expected carboxylate ion.
Mr oseltamivir = 312
-
19M.3.hl.TZ1.22b:
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.hl.TZ1.22c:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.hl.TZ1.23a:
Explain how opiates act to provide pain relief.
-
19M.3.hl.TZ1.23b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.hl.TZ1.24a(i):
Determine the percentage of technetium-99m remaining after 24.0 hours.
-
19M.3.hl.TZ1.24a(ii):
Technetium-99 decays further, emitting beta radiation. Formulate the equation for the decay of technetium-99.
-
19M.3.hl.TZ1.24b(i):
Outline what is meant by low-level waste.
-
19M.3.hl.TZ1.24b(ii):
Outline the disposal of LLW.
-
19M.3.hl.TZ1.24c:
Magnetic resonance imaging (MRI) is an application of NMR technology using radiowaves.
Suggest why MRI is much less dangerous than imaging techniques such as X-rays and radiotracers. Use section 3 of the data booklet.
-
19M.3.hl.TZ1.25a:
Identify the chiral carbon atom using an asterisk, *.
-
19M.3.hl.TZ1.25b:
Enantiomers can be identified using a polarimeter. Outline how this instrument differentiates the enantiomers.
-
19M.3.hl.TZ2.21a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
-
19M.3.hl.TZ2.21b:
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
-
19M.3.hl.TZ2.22a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.hl.TZ2.22b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.hl.TZ2.22b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.hl.TZ2.22c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.hl.TZ2.22d:
State why aspirin should not be taken with alcohol.
-
19M.3.hl.TZ2.23a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.hl.TZ2.23a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
-
19M.3.hl.TZ2.23b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19M.3.hl.TZ2.24a:
Outline one way in which antiviral drugs work.
-
19M.3.hl.TZ2.24b:
Discuss two difficulties associated with solving the AIDS problem.
-
19M.3.hl.TZ2.25a:
Examine the synthesis of taxol in terms of green chemistry criteria.
-
19M.3.hl.TZ2.25b:
Outline the operation of a polarimeter used to distinguish between enantiomers.
-
19M.3.hl.TZ2.26a:
Evaluate the suitability of technetium-99m for this use.
-
19M.3.hl.TZ2.26b:
Calculate the percentage of technetium-99m remaining after 10.0 hours. Use section 1 of the data booklet.
-
19M.3.hl.TZ2.27a:
Describe how a fuel cell breathalyser works.
-
19M.3.hl.TZ2.27b:
Alcohol levels in the breath can also be determined using IR spectroscopy.
Suggest, giving a reason, which bond’s absorbance is most useful for detecting ethanol in breath.
Bond:
Reason:
-
19M.3.sl.TZ1.14:
Aspirin can be obtained from salicylic acid.
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that differentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.sl.TZ1.15a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.sl.TZ1.15b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.sl.TZ1.15b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.sl.TZ1.16a(i):
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.sl.TZ1.16a(ii):
Determine the volume of CO2 (g), in dm3, produced at STP, when 1.00 g of CaCO3 (s) reacts completely with stomach acid.
Mr CaCO3 = 100.09
-
19M.3.sl.TZ1.16b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.sl.TZ1.17a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.sl.TZ1.17a(ii):
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.sl.TZ1.17b:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.sl.TZ1.18a:
Explain how opiates act to provide pain relief.
-
19M.3.sl.TZ1.18b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.sl.TZ1.19a:
Outline what is meant by low-level waste.
-
19M.3.sl.TZ1.19b:
Outline the disposal of LLW.
-
19M.3.sl.TZ2.14a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
-
19M.3.sl.TZ2.14b(i):
State one advantage of using morphine as an analgesic.
-
19M.3.sl.TZ2.14b(ii):
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
-
19M.3.sl.TZ2.15a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.sl.TZ2.15b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.sl.TZ2.15b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.sl.TZ2.15c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.sl.TZ2.15d:
State why aspirin should not be taken with alcohol.
-
19M.3.sl.TZ2.15e:
Outline two factors which must be considered to assess the greenness of any chemical process.
-
19M.3.sl.TZ2.16a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.sl.TZ2.16a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
-
19M.3.sl.TZ2.16b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19M.3.sl.TZ2.17a:
Outline one way in which antiviral drugs work.
-
19M.3.sl.TZ2.17b:
Discuss two difficulties associated with solving the AIDS problem.
- 19N.3.sl.TZ0.15b: Explain why diamorphine has greater potency than morphine.
- 19N.3.hl.TZ0.21b: Experimental research on both animals and humans contributes to the development...
-
19N.3.sl.TZ0.16a:
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
- 19N.3.sl.TZ0.16c: Outline how ranitidine reduces stomach acidity.
-
19N.3.sl.TZ0.16d:
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
- 19N.3.sl.TZ0.17a: Suggest one reactant used to prepare aspirin from salicylic acid.
-
19N.3.sl.TZ0.17b:
Aspirin, C6H4(OCOCH3)COOH, is only slightly soluble in water.
Outline, including an equation, how aspirin can be made more water-soluble. Use section 37 in the data booklet.
-
19N.3.hl.TZ0.24a:
Infrared (IR) spectroscopy is used to identify functional groups in organic compounds.
Deduce the wavenumber, in cm−1, of an absorption peak found in the IR spectrum of testosterone but not in that of cholesterol.
-
19N.3.hl.TZ0.24b:
Describe a technique for the detection of steroids in blood and urine.
-
19N.3.hl.TZ0.24c:
Explain how redox chemistry is used to measure the ethanol concentration in a breathalyser.
- 19N.3.hl.TZ0.25a: Explain how the beta-lactam ring is responsible for the antibiotic properties of penicillin....
- 19N.3.hl.TZ0.25b: Outline the impact of antibiotic waste on the environment.
- 19N.3.hl.TZ0.25c: Suggest a concern about the disposal of solvents from drug manufacturing.
- 19N.3.hl.TZ0.25d: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
- 19N.3.hl.TZ0.26a: State the feature of Taxol that is a major challenge in its synthesis. Use section 37 of the data...
-
19N.3.hl.TZ0.26b:
Describe how the challenge in (a) was resolved by pharmaceutical companies.
- 19N.3.hl.TZ0.27a: State two common side effects of radiotherapy.
- 19N.3.hl.TZ0.27b: Explain why technetium-99m is the most common radioisotope used in nuclear medicine.
-
19N.3.hl.TZ0.27c:
25.0 μg of iodine-131, with a half-life of 8.00 days, was left to decay.
Calculate the mass of iodine-131, in μg, remaining after 32.0 days. Use section 1 of the data booklet.
- 19N.3.sl.TZ0.15a: State the names of two functional groups present in all three molecules, using section 37 of the...
-
19N.3.sl.TZ0.16b:
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
-
19N.3.sl.TZ0.18a:
State one difference between bacteria and viruses.
- 19N.3.sl.TZ0.18b: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
- 19N.3.sl.TZ0.18c: The discovery of penicillins contributed to the development of antibiotics. Explain how the...
- 19N.3.sl.TZ0.19a: Outline the impact of antibiotic waste on the environment.
- 19N.3.sl.TZ0.19b: Suggest a concern about the disposal of solvents from drug manufacturing.
- 19N.3.hl.TZ0.21a: Explain why diamorphine has greater potency than morphine.
-
20N.3.sl.TZ0.11a:
Deduce the structural formula of the by-product of this reaction.
- 20N.3.sl.TZ0.11b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.sl.TZ0.11c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
-
20N.3.sl.TZ0.11d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
-
20N.3.sl.TZ0.12:
Consider the following antacids:
Show that antacid X is more effective, per tablet, than antacid Y.
- 20N.3.sl.TZ0.13a: Describe the proper disposal of low-level radioactive waste in hospitals.
-
20N.3.sl.TZ0.13b:
Outline a green chemistry solution for problems generated by the use of organic solvents.
- 20N.3.sl.TZ0.14a(i): Name two functional groups that both zanamivir and oseltamivir contain.
-
20N.3.sl.TZ0.14a(ii):
Explain how zanamivir works as a preventative agent against flu viruses.
- 20N.3.sl.TZ0.14b(i): Circle the side-chain in penicillin on the structure below.
-
20N.3.sl.TZ0.14b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
- 20N.3.sl.TZ0.14c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
- 20N.3.sl.TZ0.14c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
-
20N.3.hl.TZ0.15a:
Deduce the structural formula of the by-product of this reaction.
- 20N.3.hl.TZ0.15b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.hl.TZ0.15c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
-
20N.3.hl.TZ0.15d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
-
20N.3.hl.TZ0.15e:
Explain how IR spectroscopy can be used to distinguish aspirin from salicylic acid.
-
20N.3.hl.TZ0.17a:
State the type of radiation technetium-99m emits.
- 20N.3.hl.TZ0.17b: Discuss the properties that make a radioisotope suitable for diagnosis.
-
20N.3.hl.TZ0.17c:
Describe the proper disposal of low-level radioactive waste in hospitals.
-
20N.3.hl.TZ0.17d:
Technetium-99m has a half-life of hours. Calculate the amount of of technetium-99m remaining after hours.
-
20N.3.hl.TZ0.18a(ii):
The vapour pressure of pure ethanal at is .
Calculate the vapour pressure of ethanal above the liquid mixture at .
- 20N.3.hl.TZ0.18b: Describe how this mixture is separated by fractional distillation.
-
20N.3.hl.TZ0.19a:
Explain how zanamivir works as a preventative agent against flu viruses.
- 20N.3.hl.TZ0.19b(i): Circle the side-chain in penicillin on the structure below.
-
20N.3.hl.TZ0.19b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
- 20N.3.hl.TZ0.19c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
- 20N.3.hl.TZ0.19c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
- 20N.3.hl.TZ0.19d: Circle two chiral carbons in the section of the Taxol structure below.
