| Date | May 2018 | Marks available | 3 | Reference code | 18M.3.sl.TZ1.5 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Determine | Question number | 5 | Adapted from | N/A |
Question
Aluminium is produced by the electrolysis of a molten electrolyte containing bauxite.
Determine the mass, in g, of aluminium produced by the passage of a charge of 1.296 × 1013 C. Use sections 2 and 6 of the data booklet.
Markscheme
ratio of electrons : aluminium ions = 3 : 1
amount Al « » = 4.48 × 107 «mol»
mass Al «= 4.48 × 107 mol × 26.98 g mol–1» = 1.21 × 109 «g»
Award [3] for correct final answer.
[3 marks]
Examiners report
Syllabus sections
-
18M.3.sl.TZ1.4c.ii:
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
-
18M.3.sl.TZ2.3c.ii:
Calculate, to four significant figures, the concentration, in μg kg−1, of vanadium in oil giving a signal intensity of 14 950.
-
18M.3.sl.TZ2.3c.i:
Identify the purpose of each graph.
- 17N.3.sl.TZ0.4b.i: Outline why an alloy is usually harder than its components by referring to its structure.
-
19M.3.sl.TZ1.3b(ii):
Suggest a better method.
-
17M.3.sl.TZ2.6b:
Metal impurities during the production of LCoS can be analysed using ICP-MS. Each metal has a detection limit below which the uncertainty of data is too high to be valid. Suggest one factor which might influence a detection limit in ICP-MS/ICP-OES.
-
16N.3.sl.TZ0.4b:
Calculate the amount, in mol, of electrons passed using section 2 of the data booklet.
-
16N.3.sl.TZ0.4d:
Calculate the number of moles of electrons required to deposit one mole of indium. Relative atomic mass of indium, Ar=114.82.
- 18N.3.sl.TZ0.3a: State the type of particle present in the plasma formed.
- 18N.3.hl.TZ0.3a: State the type of particle present in the plasma formed.
-
17M.3.sl.TZ1.9c:
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
19M.3.hl.TZ1.3b(iii):
Suggest a better method.
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
-
19M.3.sl.TZ1.3a(ii):
Explain why lithium is paramagnetic while lithium hydride is diamagnetic by referring to electron configurations.
-
17M.3.hl.TZ2.5c.i:
Rhodium is paramagnetic with an electron configuration of [Kr] 5s14d8.
Explain, in terms of electron spin pairing, why paramagnetic substances are attracted to a magnetic field and diamagnetic substances are not.
-
16N.3.sl.TZ0.4e:
Deduce the charge on the indium ion and the formula of indium sulfate.
-
19N.3.sl.TZ0.5b(ii):
Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
-
16N.3.sl.TZ0.4c:
Calculate the mass of indium deposited by one mole of electrons.
-
19M.3.sl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48250 C. Use sections 2 and 6 of the data booklet.
-
17M.3.sl.TZ2.5c:
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
-
17M.3.hl.TZ1.10a:
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
19N.3.sl.TZ0.5a:
Discuss why different methods of reduction are needed to extract metals.
-
17M.3.sl.TZ2.5b.i:
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
-
17M.3.sl.TZ1.7a:
State why lanthanum cannot be produced by reducing its oxide with carbon.
-
19M.3.hl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.hl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
17M.3.sl.TZ1.7b:
Calculate the current (I), in A, required to produce 1.00 kg of lanthanum metal per hour. Use the formula and sections 2 and 6 of the data booklet.
-
17N.3.sl.TZ0.4c:
Explain how Inductively Coupled Plasma (ICP) Spectroscopy could be used to determine the concentration of mercury in a sample of dental filling.
-
19M.3.hl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48 250 C. Use sections 2 and 6 of the data booklet.
-
18N.3.sl.TZ0.3b:
An unknown antacid sample has a lead ion concentration of 0.50 μg dm‒3.
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
19M.3.sl.TZ1.3b(i):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
16N.3.sl.TZ0.4a:
Calculate the charge, in coulombs, passed during the electrolysis.
-
18N.3.hl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
-
18N.3.sl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
-
18M.3.sl.TZ2.3b:
ICP-MS is a reference mode for analysis. The following correlation graphs between ICP-OES and ICP-MS were produced for yttrium and nickel.
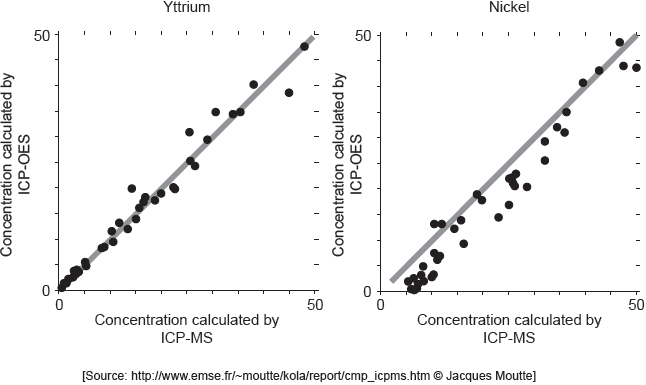
Each y-axis shows concentrations calculated by ICP-OES; each x-axis shows concentrations for the same sample as found by ICP-MS.
The line in each graph is y = x.
Discuss the effectiveness of ICP-OES for yttrium and nickel.
-
19M.3.hl.TZ1.3b(ii):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
-
20N.3.hl.TZ0.4c(iii):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
-
20N.3.sl.TZ0.4b(iv):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
-
20N.3.hl.TZ0.4c(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020
Calculate the theoretical mass of magnesium obtained if a current of 3.00 A is used for hours. Use charge :(Q) = current (I) × time (t) and section 2 of the data booklet.
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
-
20N.3.sl.TZ0.4b(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020.
Write the half-equations for the reactions occurring in this electrolysis.
-
18N.3.hl.TZ0.3b.i:
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
19M.3.sl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.sl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
20N.3.sl.TZ0.4b(iii):
Calculate the theoretical mass of magnesium obtained if a current of is used for hours. Use charge and section 2 of the data booklet

