| Date | November 2017 | Marks available | 1 | Reference code | 17N.3.sl.TZ0.7 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Deduce | Question number | 7 | Adapted from | N/A |
Question
The development of materials with unique properties is critical to advances in industry.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
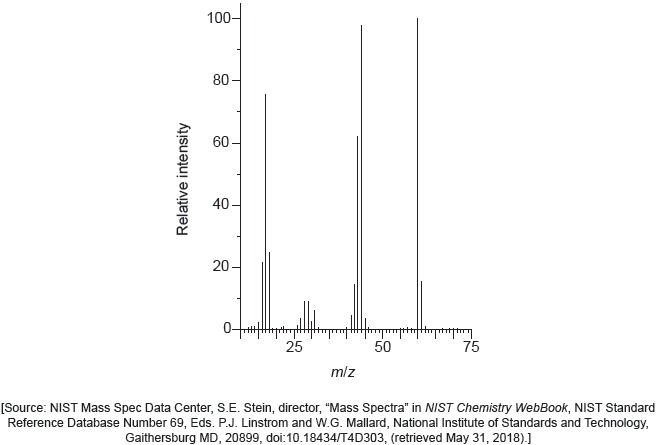
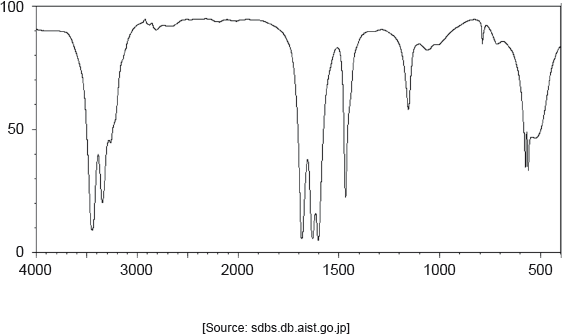
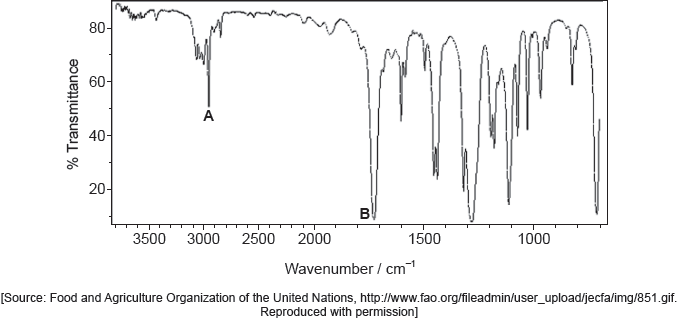
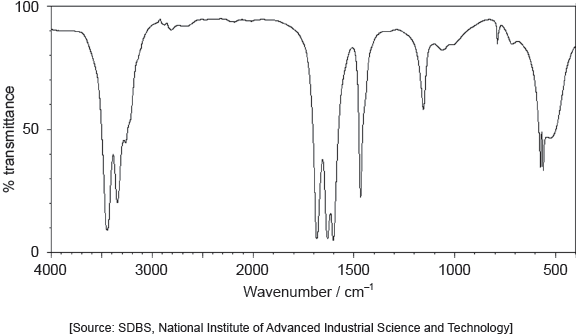
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
Markscheme
Any two of:
ability to form a LC phase
chemically stable
«LC phase that is» stable over suitable temperature range
polar
OR
being able to change orientation with applied electric field
rapid switching speed «responds to changes of voltage quickly»
Accept “ability of molecules to transmit light under certain conditions” OR “rodshaped molecules” OR “stable to light/not light sensitive”.
[Max 2 Marks]
branching in LDPE prevents close packing «of chains»
LDPE is more flexible/less rigid
OR
LDPE has lower «tensile» strength
Do not accept “difference in density”.
Award [1 max] for stating “branching in LDPE AND little/no branching in HDPE”.
B AND absence «of absorption of» C–H at 2850–3090 «cm–1»
OR
B AND presence of «absorption of» C–F at 1000–1400 «cm–1»
(–C2H3Cl–)2 (s) + 5O2 (g) → 4CO2 (g) + 2H2O (l) + 2HCl (g)
correct species in reactants and products
balanced
Accept “(–C2H3Cl–)2 (s) + 5.5O2 (g) → 4CO2 (g) + 3H2O (l) + Cl2 (g)”.
Award M2 only if M1 correct.