DP Chemistry Questionbank

D: Medicinal chemistry
| Path: |
Description
[N/A]Directly related questions
-
16N.3.sl.TZ0.20b:
Methadone is sometimes used to help reduce withdrawal symptoms in the treatment of heroin addiction. Outline one withdrawal symptom that an addict may experience.
- 16N.3.hl.TZ0.26c: Omeprazole exists as a racemic mixture whereas esomeprazole is a single enantiomer. Outline how,...
-
16N.3.hl.TZ0.28a:
Deduce equations for the following nuclear reactions:
(i) Molybdenum-98 absorbs a neutron.
(ii) The isotope produced in (a) (i) decays into technetium-99m.
- 16N.3.hl.TZ0.28b: Molybdenum-99 has a half-life of 66 hours, while technetium-99m has a half-life of 6 hours....
-
16N.3.sl.TZ0.19c:
A different approach to treating excess stomach acid is to neutralize it with antacids. Formulate an equation that shows the action of an antacid that can neutralize three moles of hydrogen ions, H+, per mole of antacid.
- 16N.3.hl.TZ0.28d: Outline the nature of the radioactive waste that is generated by the use of technetium-99m in...
- 16N.3.sl.TZ0.17a: Zanamivir must be taken by inhalation, not orally. Deduce what this suggests about the...
-
16N.3.hl.TZ0.29b:
One class of performance-enhancing drugs is the anabolic steroids. Detection of these drugs in urine samples uses a combination of gas chromatography and mass spectrometry (GC/MS).
(i) Describe how gas chromatography enables the components of urine to be analysed.
(ii) The structures of two steroids, testosterone and nandrolone, are given below.
With reference to the molar masses of the two steroids, determine, with a reason, which can be identified from the mass spectrum below.
-
16N.3.sl.TZ0.16a:
(i) Outline what is meant by the term “ring strain”.
(ii) On the diagram above, label with asterisk/s (*) the carbon atom/s that experience ring strain.
- 16N.3.hl.TZ0.28c: Outline two reasons, other than its half-life, why technetium-99m is so useful in medical diagnosis.
-
16N.3.sl.TZ0.16b:
(i) Some antibiotic-resistant bacteria produce a beta-lactamase enzyme which destroys penicillin activity. Suggest how adding clavulanic acid to penicillin enables the antibiotic to retain its activity.
(ii) Populations of antibiotic-resistant bacteria have increased significantly over the last 60 years. Outline why antibiotics such as penicillin should not be prescribed to people suffering from a viral infection.
- 16N.3.sl.TZ0.17c: The synthesis of oseltamivir is dependent on a supply of the precursor shikimic acid, which is...
- 16N.3.sl.TZ0.18d: State why aspirin is described as a mild analgesic with reference to its site of action.
-
16N.3.sl.TZ0.19a:
Ranitidine (Zantac) is a drug that inhibits stomach acid production. Outline why the development of this drug was based on a detailed knowledge of the structure of histamine, shown below.
-
16N.3.sl.TZ0.19b:
Two other drugs, omeprazole (Prilosec) and esomeprazole (Nexium), directly prevent the release of acid into the stomach. Identify the site of action in the body.
- 16N.3.hl.TZ0.29a: Suggest what may have led to these changes in acceptable concentrations.
-
20N.3.sl.TZ0.11d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
-
20N.3.sl.TZ0.14b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
-
20N.3.sl.TZ0.13b:
Outline a green chemistry solution for problems generated by the use of organic solvents.
- 20N.3.sl.TZ0.14b(i): Circle the side-chain in penicillin on the structure below.
- 20N.3.sl.TZ0.13a: Describe the proper disposal of low-level radioactive waste in hospitals.
-
20N.3.sl.TZ0.11c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
- 20N.3.sl.TZ0.14c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
- 20N.3.sl.TZ0.14c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
- 20N.3.sl.TZ0.14a(i): Name two functional groups that both zanamivir and oseltamivir contain.
-
20N.3.sl.TZ0.11a:
Deduce the structural formula of the by-product of this reaction.
- 20N.3.sl.TZ0.11b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.sl.TZ0.14a(ii):
Explain how zanamivir works as a preventative agent against flu viruses.
-
20N.3.sl.TZ0.12:
Consider the following antacids:
Show that antacid X is more effective, per tablet, than antacid Y.
- 20N.3.hl.TZ0.17b: Discuss the properties that make a radioisotope suitable for diagnosis.
- 20N.3.hl.TZ0.15b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.hl.TZ0.15c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
-
20N.3.hl.TZ0.17c:
Describe the proper disposal of low-level radioactive waste in hospitals.
-
20N.3.hl.TZ0.15e:
Explain how IR spectroscopy can be used to distinguish aspirin from salicylic acid.
-
20N.3.hl.TZ0.15d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
-
20N.3.hl.TZ0.17a:
State the type of radiation technetium-99m emits.
-
20N.3.hl.TZ0.15a:
Deduce the structural formula of the by-product of this reaction.
-
20N.3.hl.TZ0.17d:
Technetium-99m has a half-life of hours. Calculate the amount of of technetium-99m remaining after hours.
-
20N.3.hl.TZ0.18a(ii):
The vapour pressure of pure ethanal at is .
Calculate the vapour pressure of ethanal above the liquid mixture at .
-
20N.3.hl.TZ0.19a:
Explain how zanamivir works as a preventative agent against flu viruses.
- 20N.3.hl.TZ0.19c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
- 20N.3.hl.TZ0.19c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
- 20N.3.hl.TZ0.19b(i): Circle the side-chain in penicillin on the structure below.
-
20N.3.hl.TZ0.19b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
- 20N.3.hl.TZ0.19d: Circle two chiral carbons in the section of the Taxol structure below.
- 20N.3.hl.TZ0.18b: Describe how this mixture is separated by fractional distillation.
-
17M.3.sl.TZ1.18a:
Dose response curves are determined for each drug.
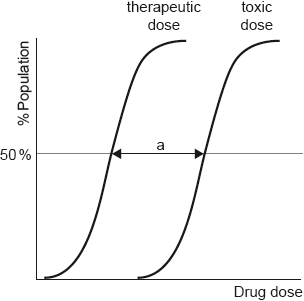
Outline the significance of range “a”.
-
17M.3.sl.TZ1.18b.i:
Suggest the type of reaction used to convert morphine to codeine.
-
17M.3.sl.TZ1.18b.ii:
State and explain the action of opiates as painkillers.
-
17M.3.sl.TZ1.19a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.sl.TZ1.19b:
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
-
17M.3.sl.TZ1.19c:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
17M.3.sl.TZ1.19d:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
17M.3.sl.TZ1.19e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
17M.3.sl.TZ1.20a:
Calculate the pH of the buffer from the following data and section 1 of the data booklet.
pKa(CO2) = 6.34
[HCO3−(aq)] = 1.40 × 10−2 moldm−3
[CO2(aq)] = 1.25 × 10−3 moldm−3
-
17M.3.sl.TZ1.20b:
Explain the effect of a large amount of aspirin on the pH of blood.
-
17M.3.sl.TZ1.21a:
Outline how oseltamivir (Tamiflu®) works.
-
17M.3.sl.TZ1.21b:
Oseltamivir was commercially produced from shikimic acid, a precursor which is a metabolite in micro-organisms and plants.
Outline how green chemistry was used to develop the precursor for oseltamivir in order to overcome a shortage of the drug during the flu season.
-
17M.3.sl.TZ1.21c:
Suggest why the administration of antibiotics to humans and animals can affect the environment.
-
17M.3.hl.TZ1.25d:
Some mild analgesics contain a solid mixture of acidic aspirin and a non-acidic organic chemical of similar polarity to asprin.
Discuss how acid-base properties and the process of solvent extraction can be used to separate aspirin from the mixture.
-
17M.3.hl.TZ1.28b:
A mixture of enantiomers shows optical rotation.
Suggest a conclusion you can draw from this data.
-
17M.3.hl.TZ1.29a:
Yttrium-90 is used in treating certain cancers.
Formulate a nuclear equation for the beta decay of yttrium-90.
-
17M.3.hl.TZ1.29b:
Lutetium-177 is a common isotope used for internal radiation therapy.
Suggest why lutetium-177 is an ideal isotope for the treatment of certain cancers based on the type of radiation emitted.
-
17M.3.hl.TZ1.29c.i:
Calculate the rate constant, , in day−1, for the decay of iodine-131 using section 1 of the data booklet.
-
17M.3.hl.TZ1.29c.ii:
Calculate the time, in days, for 90% of the sample to decay.
-
17M.3.hl.TZ1.29d:
A breathalyser measures the blood alcohol content from a breath sample. Formulate half-equations for the reactions at the anode (negative electrode) and the cathode (positive electrode) in a fuel cell breathalyser.
-
17M.3.sl.TZ2.15a.iii:
State two techniques which could be used to confirm the identity of aspirin.
-
17M.3.sl.TZ2.15b.i:
State how aspirin can be converted to water-soluble aspirin.
-
17M.3.sl.TZ2.15b.ii:
Compare, giving a reason, the bioavailability of soluble aspirin with aspirin.
-
17M.3.sl.TZ2.16a:
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
-
17M.3.sl.TZ2.16b:
Suggest a reagent used to prepare diamorphine from morphine.
-
17M.3.sl.TZ2.16c:
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
-
17M.3.sl.TZ2.17a:
Two drugs are ranitidine (Zantac) and omeprazole (Prilosec). Outline how they function to reduce stomach acidity.
-
17M.3.sl.TZ2.17b:
0.500 g of solid anhydrous sodium carbonate, Na2CO3(s), is dissolved in 75.0 cm3 of 0.100 moldm−3 sodium hydrogen carbonate solution, NaHCO3(aq). Assume the volume does not change when the salt dissolves.
HCO3−(aq) CO32−(aq) + H+(aq) pKa = 10.35.
Calculate the pH of the buffer solution.
-
17M.3.sl.TZ2.18a.i:
Compare and contrast the structures of oseltamivir and zanamivir, stating the names of functional groups.
-
17M.3.sl.TZ2.18a.ii:
Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds, using section 26 of the data booklet.
-
17M.3.sl.TZ2.18b:
Suggest one ethical consideration faced by medical researchers when developing medications.
-
17M.3.sl.TZ2.19a:
Suggest one problem associated with chlorinated organic solvents as chemical waste.
-
17M.3.sl.TZ2.19b:
Suggest how the principles of green chemistry can be used to solve the environmental problems caused by organic solvents.
-
17M.3.hl.TZ2.20a.iv:
State two techniques, other than IR spectroscopy, which could be used to confirm the identity of aspirin.
-
17M.3.hl.TZ2.22a:
Outline how ranitidine (Zantac) functions to reduce stomach acidity.
-
17M.3.hl.TZ2.25:
Taxol is produced using a chiral auxiliary. Describe how the chiral auxiliary functions to produce the desired product.
-
17M.3.hl.TZ2.26a.i:
Explain why alpha-radiation is particularly suitable for this treatment.
-
17M.3.hl.TZ2.26a.ii:
Outline how the alpha-radiation in TAT is directed to cancer cells.
-
17M.3.hl.TZ2.26b.i:
Identify the type of radiation emitted by these two radioisotopes.
-
17M.3.hl.TZ2.26b.ii:
State an equation for the one-step decay of yttrium-90.
-
17M.3.hl.TZ2.26b.iii:
The half-life of lutetium-177 is 6.75 days. Calculate the percentage remaining after 27 days.
-
17N.3.hl.TZ0.21c:
Explain the low environmental impact of most medical nuclear waste.
-
17N.3.hl.TZ0.23b:
Explain the role of the chiral auxiliary in the synthesis of Taxol.
-
17N.3.hl.TZ0.21a:
State a nuclear equation to show the decay of lutetium-177.
-
17N.3.hl.TZ0.21b:
The half-life of lutetium-177 is 6.73 days. Determine the percentage of a sample of lutetium-177 remaining after 14.0 days.
- 17N.3.hl.TZ0.22a.i: Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
-
17N.3.hl.TZ0.22a.ii:
Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section 26 of the data booklet.
- 17N.3.hl.TZ0.22b: Describe how mild analgesics function.
- 17N.3.hl.TZ0.27: Ethanol slows down the reaction time of a driver leading to traffic accidents. Explain how the...
-
17N.3.sl.TZ0.17b.ii:
Explain why opiates are addictive.
- 17N.3.sl.TZ0.17a: Aspirin is a mild analgesic derived from salicylic acid found in willow bark. Describe how mild...
-
17N.3.sl.TZ0.18a:
Outline the difference between the therapeutic index in animal studies and the therapeutic index in humans.
-
17N.3.sl.TZ0.16:
Radioisotopes are used to diagnose and treat various diseases. Explain the low environmental impact of most medical nuclear waste.
-
17N.3.sl.TZ0.19a:
State the names of two functional groups that both compounds contain, using section 37 of the data booklet.
-
17N.3.sl.TZ0.20b:
The pH is maintained in different fluids in the body by the use of buffers.
Calculate the pH of a buffer solution of 0.0200 mol dm–3 carbonic acid, H2CO3, and 0.400 mol dm–3 sodium hydrogen carbonate, NaHCO3. The pKa of carbonic acid is 6.35.
- 17N.3.sl.TZ0.18b: State the method of drug administration that gives the maximum bioavailability.
- 17N.3.sl.TZ0.19b: Explain how oseltamivir and zanamivir can stop the spread of the flu virus in the body.
-
17N.3.sl.TZ0.17b.i:
The strong analgesics morphine and codeine are opiates. Outline how codeine can be synthesized from morphine. The structures of morphine and codeine are in section 37 of the data booklet.
-
17N.3.sl.TZ0.20a:
Explain how ranitidine (Zantac) reduces stomach acid production.
- 17N.3.sl.TZ0.21: Molecules of antibiotics often contain a beta-lactam ring. Explain the importance of the...
-
18M.3.hl.TZ2.25:
Taxol was originally obtained from the bark of the Pacific yew tree.
Outline how Green Chemistry has improved the process of obtaining Taxol.
-
18M.3.hl.TZ1.16e:
Many drugs are chiral. Explain how a polarimeter can be used to determine the relative proportion of two enantiomers.
-
18M.3.hl.TZ1.19a:
Describe how ionizing radiation destroys cancer cells.
-
18M.3.hl.TZ1.19b:
Outline how Targeted Alpha Therapy (TAT) is used for treating cancers that have spread throughout the body.
-
18M.3.hl.TZ1.20a:
Hexane and propanone have vapour pressures of 17 kPa and 24 kPa respectively at 20 °C.
Calculate the vapour pressure, in kPa, at 20 °C of a mixture containing 60% hexane and 40% propanone by mole fraction, using Raoult’s law and assuming the mixture is ideal.
-
18M.3.hl.TZ1.20b:
Explain how hexane and propanone may be separated by fractional distillation.
-
18M.3.hl.TZ2.26b:
The half-life of phosphorus-32 is 14.3 days. Calculate the mass, in g, of 32P remaining after 57.2 days if the initial sample contains 2.63 × 10−8 mol. Use table 1 of the data booklet and Mr = 31.97 g mol−1.
-
18M.3.hl.TZ2.26c:
Explain the targeted alpha therapy (TAT) technique and why it is useful.
-
18M.3.hl.TZ2.26a:
Phosphorous-32 undergoes beta decay. Formulate a balanced nuclear equation for this process.
-
18M.3.hl.TZ2.27a:
Fuel cells use an electrochemical process to determine the concentration of ethanol.
Formulate the overall equation for this process.
-
18M.3.hl.TZ2.27b:
Predict the chemical shifts and integration for each signal in the 1H NMR spectrum for ethanol using section 27 of the data booklet.
-
18M.3.sl.TZ1.13d.i:
Morphine and codeine are strong analgesics. Outline how strong analgesics function.
-
18M.3.sl.TZ1.14b:
Explain how omeprazole (Prilosec) reduces stomach acidity.
-
18M.3.sl.TZ1.15b:
Shikimic acid, the precursor for oseltamivir (Tamiflu), was originally extracted from star anise, and is now produced using genetically modified E. coli bacteria.
Suggest one difficulty associated with synthesizing oseltamivir (Tamiflu) from star anise.
-
18M.3.sl.TZ1.13c.i:
Compare and contrast the IR spectrum of aspirin with that of salicylic acid, using section 26 of the data booklet.
-
18M.3.sl.TZ1.14a.i:
An antacid tablet contains 680 mg of calcium carbonate, CaCO3, and 80 mg of magnesium carbonate, MgCO3.
State the equation for the reaction of magnesium carbonate with hydrochloric acid.
-
18M.3.sl.TZ1.13a:
Aspirin is often taken to reduce pain, swelling or fever. State one other use of aspirin.
-
18M.3.sl.TZ1.13b.i:
State what is meant by the bioavailability of a drug.
-
18M.3.sl.TZ1.13b.ii:
Outline how the bioavailability of aspirin may be increased.
-
18M.3.sl.TZ1.13c.ii:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ1.13c.iii:
Outline two consequences of prescribing antibiotics such as penicillin unnecessarily.
-
18M.3.sl.TZ1.13c.iv:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ1.13d.ii:
Suggest one reason why codeine is more widely used than morphine as an analgesic.
-
18M.3.sl.TZ1.14a.ii:
Determine the amount, in mol, of hydrochloric acid neutralized by one antacid tablet.
-
18M.3.sl.TZ1.15a:
Oseltamivir (Tamiflu) and zanamivir (Relenza) are used against flu viruses. Explain how these drugs function.
-
18M.3.sl.TZ2.15:
Drug testing is necessary to determine safe and effective doses.
Distinguish between the lethal dose (LD50) and the toxic dose (TD50).
-
18M.3.sl.TZ2.16b:
State the type of reaction used to synthesize aspirin from salicylic acid.
-
18M.3.sl.TZ2.16c:
Explain why aspirin is not stored in a hot, humid location.
-
18M.3.sl.TZ2.17:
Morphine and diamorphine (heroin) are both opioids.
Explain why diamorphine is more potent than morphine using section 37 of the data booklet.
-
18M.3.sl.TZ2.20:
Drug synthesis often involves solvents.
Identify a common hazardous solvent and a Green solvent that could replace it.
-
18M.3.sl.TZ2.16a.i:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ2.16a.ii:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ2.18a:
Formulate a chemical equation for the neutralization of stomach acid with calcium carbonate.
-
18M.3.sl.TZ2.18b:
Calculate the amount, in mol, of stomach acid neutralized by an antacid tablet containing 0.750 g calcium carbonate.
-
18M.3.sl.TZ2.18c:
Explain how omeprazole (Prilosec) regulates pH in the stomach.
-
18M.3.sl.TZ2.19a:
Identify the names of two functional groups present in zanamivir using section 37 of the data booklet.
-
18M.3.sl.TZ2.19b:
Distinguish between bacteria and viruses.
-
18N.3.sl.TZ0.15a:
State one way in which viruses differ from bacteria.
- 18N.3.sl.TZ0.13c: Outline the meaning of the bioavailability of a drug.
-
18N.3.sl.TZ0.14a:
Determine the pH of a buffer solution that is 0.0100 mol dm−3 sodium hydrogen carbonate and 0.0200 mol dm−3 sodium carbonate, using section 1 of the data booklet.
Ka (hydrogen carbonate ion) = 4.8 × 10−11
-
18N.3.sl.TZ0.16:
Suggest two reasons why chlorinated solvents should neither be released into the atmosphere nor incinerated (burnt).
- 18N.3.sl.TZ0.12c: Outline one effect of over-prescription of penicillin.
- 18N.3.hl.TZ0.17b: Outline the meaning of the bioavailability of a drug.
- 18N.3.hl.TZ0.16b: Explain how the open β-lactam ring kills bacteria.
-
18N.3.sl.TZ0.14b:
State the equation for the reaction of calcium carbonate, the active ingredient in some antacids, with stomach acid.
-
18N.3.hl.TZ0.22b.ii:
Suggest why the percentage of technetium-99m remaining in the human body two days after injection will be lower than that calculated in (b)(i).
- 18N.3.sl.TZ0.12a: State the internal bond angles in the β-lactam ring and the expected bond angles for the same...
- 18N.3.sl.TZ0.12e: Suggest why human cells are not affected by penicillin.
- 18N.3.sl.TZ0.12b: Explain how the open β-lactam ring kills bacteria.
- 18N.3.sl.TZ0.12d: State how the structure of penicillin can be changed to combat this effect.
- 18N.3.sl.TZ0.14c: Suggest a technique for measuring the percentage mass of calcium carbonate in this type of...
-
18N.3.hl.TZ0.21a:
The diagram shows part of a Taxol molecule in skeletal form.
Draw a circle around each chiral carbon.
-
18N.3.sl.TZ0.13a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
- 18N.3.sl.TZ0.15b: Outline two different ways in which antiviral medications work.
- 18N.3.sl.TZ0.13b: Describe the analgesic action of an opiate.
- 18N.3.hl.TZ0.16c: State how the structure of penicillin can be modified to combat the effect of resistance caused...
-
18N.3.hl.TZ0.23a:
State an analytical technique used to separate anabolic steroids from other compounds in an athlete’s urine or blood.
-
18N.3.hl.TZ0.23b:
Ethanol in breath can be detected by a redox reaction. Outline this method of detection. An equation is not required.
-
18N.3.hl.TZ0.17a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
-
18N.3.hl.TZ0.22a:
Alpha particles are more damaging to human cells than any other nuclear radiation and yet they are used in targeted alpha therapy (TAT).
Explain how TAT is relatively safe to use in the treatment of dispersed cancers.
- 18N.3.hl.TZ0.16a: State the internal bond angles in the b-lactam ring and the expected bond angles in sp2 and sp3...
- 18N.3.hl.TZ0.16d: Suggest why human cells are not affected by penicillin.
- 18N.3.hl.TZ0.19: Outline two different ways in which antiviral medications work.
- 18N.3.hl.TZ0.21b: Outline how chiral auxiliaries are used to synthesize the desired enantiomer.
- 18N.3.hl.TZ0.21c: Explain the process of solvent extraction by which Taxol is isolated.
-
18N.3.hl.TZ0.22b.i:
Technetium-99m () has a half-life of 6.0 hours. Calculate the percentage of remaining in a sample of the radioisotope after two days.
-
17M.3.hl.TZ1.25c:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
17M.3.hl.TZ1.25a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.hl.TZ1.25e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
17M.3.hl.TZ1.25b:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
19M.3.hl.TZ1.21a:
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.hl.TZ1.24a(ii):
Technetium-99 decays further, emitting beta radiation. Formulate the equation for the decay of technetium-99.
-
19M.3.hl.TZ1.24b(i):
Outline what is meant by low-level waste.
-
19M.3.hl.TZ1.23a:
Explain how opiates act to provide pain relief.
-
19M.3.hl.TZ1.22c:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.hl.TZ1.22a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.hl.TZ1.25a:
Identify the chiral carbon atom using an asterisk, *.
-
19M.3.hl.TZ1.22b:
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.hl.TZ1.25b:
Enantiomers can be identified using a polarimeter. Outline how this instrument differentiates the enantiomers.
-
19M.3.hl.TZ1.24b(ii):
Outline the disposal of LLW.
-
19M.3.hl.TZ1.24c:
Magnetic resonance imaging (MRI) is an application of NMR technology using radiowaves.
Suggest why MRI is much less dangerous than imaging techniques such as X-rays and radiotracers. Use section 3 of the data booklet.
-
19M.3.hl.TZ1.19a:
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that diff erentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.hl.TZ1.24a(i):
Determine the percentage of technetium-99m remaining after 24.0 hours.
-
19M.3.hl.TZ1.22a(ii):
The resulting active metabolite of oseltamivir can be detected by mass spectrometry (MS) analysis.
Deduce the mass of the expected carboxylate ion.
Mr oseltamivir = 312
-
19M.3.hl.TZ2.22b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.hl.TZ1.20a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.hl.TZ1.20b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.hl.TZ1.20b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.hl.TZ1.23b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.hl.TZ1.21b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.hl.TZ2.22c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.hl.TZ2.21b:
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
-
19M.3.hl.TZ2.25a:
Examine the synthesis of taxol in terms of green chemistry criteria.
-
19M.3.hl.TZ2.27a:
Describe how a fuel cell breathalyser works.
-
19M.3.hl.TZ2.26a:
Evaluate the suitability of technetium-99m for this use.
-
19M.3.hl.TZ2.26b:
Calculate the percentage of technetium-99m remaining after 10.0 hours. Use section 1 of the data booklet.
-
19M.3.hl.TZ2.23b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19M.3.hl.TZ2.24b:
Discuss two difficulties associated with solving the AIDS problem.
-
19M.3.hl.TZ2.22a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.hl.TZ2.22b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.hl.TZ2.23a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.hl.TZ2.23a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
-
19M.3.hl.TZ2.25b:
Outline the operation of a polarimeter used to distinguish between enantiomers.
-
19M.3.hl.TZ2.21a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
-
19M.3.hl.TZ2.27b:
Alcohol levels in the breath can also be determined using IR spectroscopy.
Suggest, giving a reason, which bond’s absorbance is most useful for detecting ethanol in breath.
Bond:
Reason:
-
19M.3.hl.TZ2.22d:
State why aspirin should not be taken with alcohol.
-
19M.3.hl.TZ2.24a:
Outline one way in which antiviral drugs work.
-
19M.3.sl.TZ1.14:
Aspirin can be obtained from salicylic acid.
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that differentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.sl.TZ1.19b:
Outline the disposal of LLW.
-
19M.3.sl.TZ1.19a:
Outline what is meant by low-level waste.
-
19M.3.sl.TZ1.16a(ii):
Determine the volume of CO2 (g), in dm3, produced at STP, when 1.00 g of CaCO3 (s) reacts completely with stomach acid.
Mr CaCO3 = 100.09
-
19M.3.sl.TZ1.18a:
Explain how opiates act to provide pain relief.
-
19M.3.sl.TZ1.18b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.sl.TZ1.16b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.sl.TZ1.17a(ii):
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.sl.TZ1.15b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.sl.TZ1.17b:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.sl.TZ1.16a(i):
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.sl.TZ1.15a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.sl.TZ1.17a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.sl.TZ1.15b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.sl.TZ2.14b(i):
State one advantage of using morphine as an analgesic.
-
19M.3.sl.TZ2.15a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.sl.TZ2.14a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
-
19M.3.sl.TZ2.15c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.sl.TZ2.16b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19M.3.sl.TZ2.14b(ii):
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
-
19M.3.sl.TZ2.15b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.sl.TZ2.16a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.sl.TZ2.17a:
Outline one way in which antiviral drugs work.
-
19M.3.sl.TZ2.17b:
Discuss two difficulties associated with solving the AIDS problem.
-
19M.3.sl.TZ2.15e:
Outline two factors which must be considered to assess the greenness of any chemical process.
-
19M.3.sl.TZ2.15b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.sl.TZ2.16a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
-
19M.3.sl.TZ2.15d:
State why aspirin should not be taken with alcohol.
- 19N.3.sl.TZ0.17a: Suggest one reactant used to prepare aspirin from salicylic acid.
- 19N.3.sl.TZ0.19b: Suggest a concern about the disposal of solvents from drug manufacturing.
-
19N.3.sl.TZ0.16d:
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
- 19N.3.hl.TZ0.27a: State two common side effects of radiotherapy.
- 19N.3.sl.TZ0.15b: Explain why diamorphine has greater potency than morphine.
- 19N.3.hl.TZ0.27b: Explain why technetium-99m is the most common radioisotope used in nuclear medicine.
-
19N.3.sl.TZ0.16b:
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
-
19N.3.sl.TZ0.17b:
Aspirin, C6H4(OCOCH3)COOH, is only slightly soluble in water.
Outline, including an equation, how aspirin can be made more water-soluble. Use section 37 in the data booklet.
- 19N.3.sl.TZ0.18b: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
- 19N.3.sl.TZ0.18c: The discovery of penicillins contributed to the development of antibiotics. Explain how the...
- 19N.3.hl.TZ0.25b: Outline the impact of antibiotic waste on the environment.
-
19N.3.hl.TZ0.26b:
Describe how the challenge in (a) was resolved by pharmaceutical companies.
- 19N.3.sl.TZ0.15a: State the names of two functional groups present in all three molecules, using section 37 of the...
- 19N.3.hl.TZ0.25c: Suggest a concern about the disposal of solvents from drug manufacturing.
- 19N.3.hl.TZ0.25d: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
-
19N.3.hl.TZ0.24c:
Explain how redox chemistry is used to measure the ethanol concentration in a breathalyser.
- 19N.3.hl.TZ0.26a: State the feature of Taxol that is a major challenge in its synthesis. Use section 37 of the data...
- 19N.3.sl.TZ0.19a: Outline the impact of antibiotic waste on the environment.
-
19N.3.hl.TZ0.24b:
Describe a technique for the detection of steroids in blood and urine.
- 19N.3.hl.TZ0.21b: Experimental research on both animals and humans contributes to the development...
-
19N.3.sl.TZ0.18a:
State one difference between bacteria and viruses.
-
19N.3.hl.TZ0.27c:
25.0 μg of iodine-131, with a half-life of 8.00 days, was left to decay.
Calculate the mass of iodine-131, in μg, remaining after 32.0 days. Use section 1 of the data booklet.
-
19N.3.hl.TZ0.24a:
Infrared (IR) spectroscopy is used to identify functional groups in organic compounds.
Deduce the wavenumber, in cm−1, of an absorption peak found in the IR spectrum of testosterone but not in that of cholesterol.
- 19N.3.sl.TZ0.16c: Outline how ranitidine reduces stomach acidity.
-
19N.3.sl.TZ0.16a:
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
- 19N.3.hl.TZ0.21a: Explain why diamorphine has greater potency than morphine.
- 19N.3.hl.TZ0.25a: Explain how the beta-lactam ring is responsible for the antibiotic properties of penicillin....
Sub sections and their related questions
D.1 Pharmaceutical products and drug action
-
17M.3.sl.TZ1.18a:
Dose response curves are determined for each drug.

Outline the significance of range “a”.
-
17M.3.sl.TZ2.15b.ii:
Compare, giving a reason, the bioavailability of soluble aspirin with aspirin.
-
17M.3.sl.TZ2.18b:
Suggest one ethical consideration faced by medical researchers when developing medications.
-
17N.3.sl.TZ0.18a:
Outline the difference between the therapeutic index in animal studies and the therapeutic index in humans.
- 17N.3.sl.TZ0.18b: State the method of drug administration that gives the maximum bioavailability.
-
18M.3.sl.TZ1.13b.i:
State what is meant by the bioavailability of a drug.
-
18M.3.sl.TZ2.15:
Drug testing is necessary to determine safe and effective doses.
Distinguish between the lethal dose (LD50) and the toxic dose (TD50).
- 18N.3.sl.TZ0.12a: State the internal bond angles in the β-lactam ring and the expected bond angles for the same...
- 18N.3.sl.TZ0.13c: Outline the meaning of the bioavailability of a drug.
- 18N.3.hl.TZ0.17b: Outline the meaning of the bioavailability of a drug.
-
19M.3.hl.TZ1.22b:
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.hl.TZ2.21a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
-
19M.3.sl.TZ1.17a(ii):
Suggest a reason for using a phosphate salt of oseltamivir in oral tablets.
-
19M.3.sl.TZ2.14a:
Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
- 19N.3.hl.TZ0.21b: Experimental research on both animals and humans contributes to the development...
-
20N.3.sl.TZ0.11d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
-
20N.3.sl.TZ0.12:
Consider the following antacids:
Show that antacid X is more effective, per tablet, than antacid Y.
-
20N.3.hl.TZ0.15d:
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
D.2 Aspirin and penicillin
-
16N.3.sl.TZ0.16a:
(i) Outline what is meant by the term “ring strain”.
(ii) On the diagram above, label with asterisk/s (*) the carbon atom/s that experience ring strain.
-
16N.3.sl.TZ0.16b:
(i) Some antibiotic-resistant bacteria produce a beta-lactamase enzyme which destroys penicillin activity. Suggest how adding clavulanic acid to penicillin enables the antibiotic to retain its activity.
(ii) Populations of antibiotic-resistant bacteria have increased significantly over the last 60 years. Outline why antibiotics such as penicillin should not be prescribed to people suffering from a viral infection.
- 16N.3.sl.TZ0.18d: State why aspirin is described as a mild analgesic with reference to its site of action.
-
17M.3.sl.TZ1.19a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.sl.TZ1.19b:
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
-
17M.3.sl.TZ1.19c:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
17M.3.sl.TZ1.19d:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
17M.3.sl.TZ2.15a.iii:
State two techniques which could be used to confirm the identity of aspirin.
-
17M.3.sl.TZ2.15b.i:
State how aspirin can be converted to water-soluble aspirin.
-
17M.3.hl.TZ2.20a.iv:
State two techniques, other than IR spectroscopy, which could be used to confirm the identity of aspirin.
- 17N.3.sl.TZ0.17a: Aspirin is a mild analgesic derived from salicylic acid found in willow bark. Describe how mild...
- 17N.3.sl.TZ0.21: Molecules of antibiotics often contain a beta-lactam ring. Explain the importance of the...
- 17N.3.hl.TZ0.22b: Describe how mild analgesics function.
-
18M.3.sl.TZ1.13a:
Aspirin is often taken to reduce pain, swelling or fever. State one other use of aspirin.
-
18M.3.sl.TZ1.13b.ii:
Outline how the bioavailability of aspirin may be increased.
-
18M.3.sl.TZ1.13c.i:
Compare and contrast the IR spectrum of aspirin with that of salicylic acid, using section 26 of the data booklet.
-
18M.3.sl.TZ1.13c.ii:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ1.13c.iv:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ2.16a.i:
Describe how penicillin combats bacterial infections.
-
18M.3.sl.TZ2.16a.ii:
State how penicillins may be modified to increase their effectiveness.
-
18M.3.sl.TZ2.16b:
State the type of reaction used to synthesize aspirin from salicylic acid.
-
18M.3.sl.TZ2.16c:
Explain why aspirin is not stored in a hot, humid location.
- 18N.3.sl.TZ0.12a: State the internal bond angles in the β-lactam ring and the expected bond angles for the same...
- 18N.3.sl.TZ0.12b: Explain how the open β-lactam ring kills bacteria.
- 18N.3.sl.TZ0.12d: State how the structure of penicillin can be changed to combat this effect.
- 18N.3.sl.TZ0.12e: Suggest why human cells are not affected by penicillin.
- 18N.3.hl.TZ0.16a: State the internal bond angles in the b-lactam ring and the expected bond angles in sp2 and sp3...
- 18N.3.hl.TZ0.16b: Explain how the open β-lactam ring kills bacteria.
- 18N.3.hl.TZ0.16c: State how the structure of penicillin can be modified to combat the effect of resistance caused...
- 18N.3.hl.TZ0.16d: Suggest why human cells are not affected by penicillin.
-
17M.3.hl.TZ1.25a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.3.hl.TZ1.25b:
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
-
17M.3.hl.TZ1.25c:
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
-
19M.3.hl.TZ1.19a:
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that diff erentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.hl.TZ1.20a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.hl.TZ1.20b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.hl.TZ1.20b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.hl.TZ2.22a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.hl.TZ2.22b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.hl.TZ2.22b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.hl.TZ2.22c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.hl.TZ2.22d:
State why aspirin should not be taken with alcohol.
-
19M.3.sl.TZ1.14:
Aspirin can be obtained from salicylic acid.
Unreacted salicylic acid may be present as an impurity in aspirin and can be detected in the infrared (IR) spectrum.
Name the functional group and identify the absorption band that differentiates salicylic acid from aspirin. Use section 26 of the data booklet.
Name:
Absorption band:
-
19M.3.sl.TZ1.15a:
Identify the feature in penicillin responsible for its antibiotic activity.
-
19M.3.sl.TZ1.15b(i):
The widespread use of penicillin and its derivatives has led to the appearance of resistant S. aureus strains.
Outline how these bacteria inactivate the antibiotics.
-
19M.3.sl.TZ1.15b(ii):
Outline how the structure of penicillin has been modified to overcome this resistance.
-
19M.3.sl.TZ2.15a:
Predict one absorption band present in an infrared (IR) spectrum of aspirin, using section 26 of the data booklet.
-
19M.3.sl.TZ2.15b(i):
Determine the mass of aspirin which reacted with 16.25 cm3 of 0.100 mol dm−3 NaOH solution.
-
19M.3.sl.TZ2.15b(ii):
Determine the percentage purity of the synthesized aspirin.
-
19M.3.sl.TZ2.15c:
Outline how aspirin can be chemically modified to increase its solubility in water.
-
19M.3.sl.TZ2.15d:
State why aspirin should not be taken with alcohol.
- 19N.3.sl.TZ0.17a: Suggest one reactant used to prepare aspirin from salicylic acid.
-
19N.3.sl.TZ0.17b:
Aspirin, C6H4(OCOCH3)COOH, is only slightly soluble in water.
Outline, including an equation, how aspirin can be made more water-soluble. Use section 37 in the data booklet.
- 19N.3.hl.TZ0.25a: Explain how the beta-lactam ring is responsible for the antibiotic properties of penicillin....
- 19N.3.sl.TZ0.18c: The discovery of penicillins contributed to the development of antibiotics. Explain how the...
-
20N.3.sl.TZ0.11a:
Deduce the structural formula of the by-product of this reaction.
- 20N.3.sl.TZ0.11b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.sl.TZ0.11c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
- 20N.3.sl.TZ0.14b(i): Circle the side-chain in penicillin on the structure below.
-
20N.3.sl.TZ0.14b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
-
20N.3.hl.TZ0.15a:
Deduce the structural formula of the by-product of this reaction.
- 20N.3.hl.TZ0.15b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.hl.TZ0.15c:
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
-
20N.3.hl.TZ0.15e:
Explain how IR spectroscopy can be used to distinguish aspirin from salicylic acid.
- 20N.3.hl.TZ0.19b(i): Circle the side-chain in penicillin on the structure below.
-
20N.3.hl.TZ0.19b(ii):
Explain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
D.3 Opiates
-
16N.3.sl.TZ0.20b:
Methadone is sometimes used to help reduce withdrawal symptoms in the treatment of heroin addiction. Outline one withdrawal symptom that an addict may experience.
-
17M.3.sl.TZ1.18b.i:
Suggest the type of reaction used to convert morphine to codeine.
-
17M.3.sl.TZ1.18b.ii:
State and explain the action of opiates as painkillers.
-
17M.3.sl.TZ2.16a:
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
-
17M.3.sl.TZ2.16b:
Suggest a reagent used to prepare diamorphine from morphine.
-
17M.3.sl.TZ2.16c:
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
-
17N.3.sl.TZ0.17b.i:
The strong analgesics morphine and codeine are opiates. Outline how codeine can be synthesized from morphine. The structures of morphine and codeine are in section 37 of the data booklet.
-
17N.3.sl.TZ0.17b.ii:
Explain why opiates are addictive.
-
18M.3.sl.TZ1.13d.i:
Morphine and codeine are strong analgesics. Outline how strong analgesics function.
-
18M.3.sl.TZ1.13d.ii:
Suggest one reason why codeine is more widely used than morphine as an analgesic.
-
18M.3.sl.TZ2.17:
Morphine and diamorphine (heroin) are both opioids.
Explain why diamorphine is more potent than morphine using section 37 of the data booklet.
-
18N.3.sl.TZ0.13a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
- 18N.3.sl.TZ0.13b: Describe the analgesic action of an opiate.
-
18N.3.hl.TZ0.17a:
Explain why diamorphine (heroin) crosses the blood–brain barrier more easily than morphine.
-
19M.3.hl.TZ1.23a:
Explain how opiates act to provide pain relief.
-
19M.3.hl.TZ1.23b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.hl.TZ2.21b:
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
-
19M.3.sl.TZ1.18a:
Explain how opiates act to provide pain relief.
-
19M.3.sl.TZ1.18b:
Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood–brain barrier. Use section 37 of the data booklet.
-
19M.3.sl.TZ2.14b(i):
State one advantage of using morphine as an analgesic.
-
19M.3.sl.TZ2.14b(ii):
Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
- 19N.3.sl.TZ0.15b: Explain why diamorphine has greater potency than morphine.
- 19N.3.sl.TZ0.15a: State the names of two functional groups present in all three molecules, using section 37 of the...
- 19N.3.hl.TZ0.21a: Explain why diamorphine has greater potency than morphine.
- 20N.3.sl.TZ0.14c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
- 20N.3.sl.TZ0.14c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
- 20N.3.hl.TZ0.19c(i): State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
- 20N.3.hl.TZ0.19c(ii): State the natural source from which codeine, morphine and diamorphine are obtained.
D.4 pH regulation of the stomach
-
16N.3.sl.TZ0.19a:
Ranitidine (Zantac) is a drug that inhibits stomach acid production. Outline why the development of this drug was based on a detailed knowledge of the structure of histamine, shown below.
-
16N.3.sl.TZ0.19b:
Two other drugs, omeprazole (Prilosec) and esomeprazole (Nexium), directly prevent the release of acid into the stomach. Identify the site of action in the body.
-
16N.3.sl.TZ0.19c:
A different approach to treating excess stomach acid is to neutralize it with antacids. Formulate an equation that shows the action of an antacid that can neutralize three moles of hydrogen ions, H+, per mole of antacid.
-
17M.3.sl.TZ1.20a:
Calculate the pH of the buffer from the following data and section 1 of the data booklet.
pKa(CO2) = 6.34
[HCO3−(aq)] = 1.40 × 10−2 moldm−3
[CO2(aq)] = 1.25 × 10−3 moldm−3
-
17M.3.sl.TZ1.20b:
Explain the effect of a large amount of aspirin on the pH of blood.
-
17M.3.sl.TZ2.17a:
Two drugs are ranitidine (Zantac) and omeprazole (Prilosec). Outline how they function to reduce stomach acidity.
-
17M.3.sl.TZ2.17b:
0.500 g of solid anhydrous sodium carbonate, Na2CO3(s), is dissolved in 75.0 cm3 of 0.100 moldm−3 sodium hydrogen carbonate solution, NaHCO3(aq). Assume the volume does not change when the salt dissolves.
HCO3−(aq) CO32−(aq) + H+(aq) pKa = 10.35.
Calculate the pH of the buffer solution.
-
17M.3.sl.TZ2.18a.i:
Compare and contrast the structures of oseltamivir and zanamivir, stating the names of functional groups.
-
17M.3.sl.TZ2.18a.ii:
Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds, using section 26 of the data booklet.
-
17M.3.hl.TZ2.22a:
Outline how ranitidine (Zantac) functions to reduce stomach acidity.
-
17N.3.sl.TZ0.20a:
Explain how ranitidine (Zantac) reduces stomach acid production.
-
17N.3.sl.TZ0.20b:
The pH is maintained in different fluids in the body by the use of buffers.
Calculate the pH of a buffer solution of 0.0200 mol dm–3 carbonic acid, H2CO3, and 0.400 mol dm–3 sodium hydrogen carbonate, NaHCO3. The pKa of carbonic acid is 6.35.
-
18M.3.sl.TZ1.14a.i:
An antacid tablet contains 680 mg of calcium carbonate, CaCO3, and 80 mg of magnesium carbonate, MgCO3.
State the equation for the reaction of magnesium carbonate with hydrochloric acid.
-
18M.3.sl.TZ1.14a.ii:
Determine the amount, in mol, of hydrochloric acid neutralized by one antacid tablet.
-
18M.3.sl.TZ1.14b:
Explain how omeprazole (Prilosec) reduces stomach acidity.
-
18M.3.sl.TZ2.18a:
Formulate a chemical equation for the neutralization of stomach acid with calcium carbonate.
-
18M.3.sl.TZ2.18b:
Calculate the amount, in mol, of stomach acid neutralized by an antacid tablet containing 0.750 g calcium carbonate.
-
18M.3.sl.TZ2.18c:
Explain how omeprazole (Prilosec) regulates pH in the stomach.
-
18N.3.sl.TZ0.14a:
Determine the pH of a buffer solution that is 0.0100 mol dm−3 sodium hydrogen carbonate and 0.0200 mol dm−3 sodium carbonate, using section 1 of the data booklet.
Ka (hydrogen carbonate ion) = 4.8 × 10−11
-
18N.3.sl.TZ0.14b:
State the equation for the reaction of calcium carbonate, the active ingredient in some antacids, with stomach acid.
- 18N.3.sl.TZ0.14c: Suggest a technique for measuring the percentage mass of calcium carbonate in this type of...
-
19M.3.hl.TZ1.21a:
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.hl.TZ1.21b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.hl.TZ2.23a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.hl.TZ2.23a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
-
19M.3.hl.TZ2.23b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19M.3.sl.TZ1.16a(i):
Formulate an equation for the neutralization of stomach acid with calcium carbonate, CaCO3 (s).
-
19M.3.sl.TZ1.16a(ii):
Determine the volume of CO2 (g), in dm3, produced at STP, when 1.00 g of CaCO3 (s) reacts completely with stomach acid.
Mr CaCO3 = 100.09
-
19M.3.sl.TZ1.16b:
Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
-
19M.3.sl.TZ2.16a(i):
Outline how ranitidine (Zantac) inhibits stomach acid production.
-
19M.3.sl.TZ2.16a(ii):
Outline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
-
19M.3.sl.TZ2.16b:
Some antacids contain carbonates.
Determine the pH of a buffer solution which contains 0.160 mol dm−3 CO32− and 0.200 mol dm−3 HCO3−, using section 1 of the data booklet.
pKa (HCO3−) = 10.32
-
19N.3.sl.TZ0.16a:
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
- 19N.3.sl.TZ0.16c: Outline how ranitidine reduces stomach acidity.
-
19N.3.sl.TZ0.16d:
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
-
19N.3.sl.TZ0.16b:
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
D.5 Anti-viral medications
-
16N.3.sl.TZ0.16b:
(i) Some antibiotic-resistant bacteria produce a beta-lactamase enzyme which destroys penicillin activity. Suggest how adding clavulanic acid to penicillin enables the antibiotic to retain its activity.
(ii) Populations of antibiotic-resistant bacteria have increased significantly over the last 60 years. Outline why antibiotics such as penicillin should not be prescribed to people suffering from a viral infection.
- 16N.3.sl.TZ0.17a: Zanamivir must be taken by inhalation, not orally. Deduce what this suggests about the...
-
17M.3.sl.TZ1.21a:
Outline how oseltamivir (Tamiflu®) works.
-
17N.3.sl.TZ0.19a:
State the names of two functional groups that both compounds contain, using section 37 of the data booklet.
- 17N.3.sl.TZ0.19b: Explain how oseltamivir and zanamivir can stop the spread of the flu virus in the body.
-
18M.3.sl.TZ1.15a:
Oseltamivir (Tamiflu) and zanamivir (Relenza) are used against flu viruses. Explain how these drugs function.
-
18M.3.sl.TZ2.19a:
Identify the names of two functional groups present in zanamivir using section 37 of the data booklet.
-
18M.3.sl.TZ2.19b:
Distinguish between bacteria and viruses.
-
18N.3.sl.TZ0.15a:
State one way in which viruses differ from bacteria.
- 18N.3.sl.TZ0.15b: Outline two different ways in which antiviral medications work.
- 18N.3.hl.TZ0.19: Outline two different ways in which antiviral medications work.
-
19M.3.hl.TZ1.22a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.hl.TZ1.22c:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.hl.TZ2.24a:
Outline one way in which antiviral drugs work.
-
19M.3.hl.TZ2.24b:
Discuss two difficulties associated with solving the AIDS problem.
-
19M.3.sl.TZ1.17a(i):
Draw a circle around the functional group that can be converted to the carboxylate by hydrolysis.
-
19M.3.sl.TZ1.17b:
Anti-HIV drugs, such as zidovudine, often become less effective over time.
Explain the development of resistant virus strains in the presence of antiviral drugs.
-
19M.3.sl.TZ2.17a:
Outline one way in which antiviral drugs work.
-
19M.3.sl.TZ2.17b:
Discuss two difficulties associated with solving the AIDS problem.
- 19N.3.hl.TZ0.25d: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
-
19N.3.sl.TZ0.18a:
State one difference between bacteria and viruses.
- 19N.3.sl.TZ0.18b: Discuss two difficulties, apart from socio-economic factors, associated with finding a cure for...
- 20N.3.sl.TZ0.14a(i): Name two functional groups that both zanamivir and oseltamivir contain.
-
20N.3.sl.TZ0.14a(ii):
Explain how zanamivir works as a preventative agent against flu viruses.
-
20N.3.hl.TZ0.19a:
Explain how zanamivir works as a preventative agent against flu viruses.
D.6 Environmental impact of some medications
- 16N.3.sl.TZ0.17c: The synthesis of oseltamivir is dependent on a supply of the precursor shikimic acid, which is...
- 16N.3.hl.TZ0.28d: Outline the nature of the radioactive waste that is generated by the use of technetium-99m in...
-
17M.3.sl.TZ1.19e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
17M.3.sl.TZ1.21b:
Oseltamivir was commercially produced from shikimic acid, a precursor which is a metabolite in micro-organisms and plants.
Outline how green chemistry was used to develop the precursor for oseltamivir in order to overcome a shortage of the drug during the flu season.
-
17M.3.sl.TZ1.21c:
Suggest why the administration of antibiotics to humans and animals can affect the environment.
-
17M.3.sl.TZ2.19a:
Suggest one problem associated with chlorinated organic solvents as chemical waste.
-
17M.3.sl.TZ2.19b:
Suggest how the principles of green chemistry can be used to solve the environmental problems caused by organic solvents.
-
17N.3.sl.TZ0.16:
Radioisotopes are used to diagnose and treat various diseases. Explain the low environmental impact of most medical nuclear waste.
-
17N.3.hl.TZ0.21c:
Explain the low environmental impact of most medical nuclear waste.
-
18M.3.sl.TZ1.13c.iii:
Outline two consequences of prescribing antibiotics such as penicillin unnecessarily.
-
18M.3.sl.TZ1.15b:
Shikimic acid, the precursor for oseltamivir (Tamiflu), was originally extracted from star anise, and is now produced using genetically modified E. coli bacteria.
Suggest one difficulty associated with synthesizing oseltamivir (Tamiflu) from star anise.
-
18M.3.sl.TZ2.20:
Drug synthesis often involves solvents.
Identify a common hazardous solvent and a Green solvent that could replace it.
- 18N.3.sl.TZ0.12c: Outline one effect of over-prescription of penicillin.
-
18N.3.sl.TZ0.16:
Suggest two reasons why chlorinated solvents should neither be released into the atmosphere nor incinerated (burnt).
-
17M.3.hl.TZ1.25e:
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
-
19M.3.hl.TZ1.24b(i):
Outline what is meant by low-level waste.
-
19M.3.hl.TZ2.25a:
Examine the synthesis of taxol in terms of green chemistry criteria.
-
19M.3.sl.TZ1.19a:
Outline what is meant by low-level waste.
-
19M.3.sl.TZ1.19b:
Outline the disposal of LLW.
-
19M.3.sl.TZ2.15e:
Outline two factors which must be considered to assess the greenness of any chemical process.
- 19N.3.hl.TZ0.25b: Outline the impact of antibiotic waste on the environment.
- 19N.3.hl.TZ0.25c: Suggest a concern about the disposal of solvents from drug manufacturing.
- 19N.3.sl.TZ0.19a: Outline the impact of antibiotic waste on the environment.
- 19N.3.sl.TZ0.19b: Suggest a concern about the disposal of solvents from drug manufacturing.
- 20N.3.sl.TZ0.13a: Describe the proper disposal of low-level radioactive waste in hospitals.
-
20N.3.sl.TZ0.13b:
Outline a green chemistry solution for problems generated by the use of organic solvents.
-
20N.3.hl.TZ0.17c:
Describe the proper disposal of low-level radioactive waste in hospitals.
D.7 Taxol—a chiral auxiliary case study (HL only)
- 16N.3.hl.TZ0.26c: Omeprazole exists as a racemic mixture whereas esomeprazole is a single enantiomer. Outline how,...
-
17M.3.hl.TZ1.28b:
A mixture of enantiomers shows optical rotation.
Suggest a conclusion you can draw from this data.
-
17M.3.hl.TZ2.25:
Taxol is produced using a chiral auxiliary. Describe how the chiral auxiliary functions to produce the desired product.
-
17N.3.hl.TZ0.23b:
Explain the role of the chiral auxiliary in the synthesis of Taxol.
-
18M.3.hl.TZ1.16e:
Many drugs are chiral. Explain how a polarimeter can be used to determine the relative proportion of two enantiomers.
-
18M.3.hl.TZ2.25:
Taxol was originally obtained from the bark of the Pacific yew tree.
Outline how Green Chemistry has improved the process of obtaining Taxol.
-
18N.3.hl.TZ0.21a:
The diagram shows part of a Taxol molecule in skeletal form.
Draw a circle around each chiral carbon.
- 18N.3.hl.TZ0.21b: Outline how chiral auxiliaries are used to synthesize the desired enantiomer.
- 18N.3.hl.TZ0.21c: Explain the process of solvent extraction by which Taxol is isolated.
-
19M.3.hl.TZ1.25a:
Identify the chiral carbon atom using an asterisk, *.
-
19M.3.hl.TZ1.25b:
Enantiomers can be identified using a polarimeter. Outline how this instrument differentiates the enantiomers.
-
19M.3.hl.TZ2.25b:
Outline the operation of a polarimeter used to distinguish between enantiomers.
- 19N.3.hl.TZ0.26a: State the feature of Taxol that is a major challenge in its synthesis. Use section 37 of the data...
-
19N.3.hl.TZ0.26b:
Describe how the challenge in (a) was resolved by pharmaceutical companies.
- 20N.3.hl.TZ0.19d: Circle two chiral carbons in the section of the Taxol structure below.
D.8 Nuclear medicine (HL only)
-
16N.3.hl.TZ0.28a:
Deduce equations for the following nuclear reactions:
(i) Molybdenum-98 absorbs a neutron.
(ii) The isotope produced in (a) (i) decays into technetium-99m.
- 16N.3.hl.TZ0.28b: Molybdenum-99 has a half-life of 66 hours, while technetium-99m has a half-life of 6 hours....
- 16N.3.hl.TZ0.28c: Outline two reasons, other than its half-life, why technetium-99m is so useful in medical diagnosis.
-
17M.3.hl.TZ1.29a:
Yttrium-90 is used in treating certain cancers.
Formulate a nuclear equation for the beta decay of yttrium-90.
-
17M.3.hl.TZ1.29b:
Lutetium-177 is a common isotope used for internal radiation therapy.
Suggest why lutetium-177 is an ideal isotope for the treatment of certain cancers based on the type of radiation emitted.
-
17M.3.hl.TZ1.29c.i:
Calculate the rate constant, , in day−1, for the decay of iodine-131 using section 1 of the data booklet.
-
17M.3.hl.TZ1.29c.ii:
Calculate the time, in days, for 90% of the sample to decay.
-
17M.3.hl.TZ2.26a.i:
Explain why alpha-radiation is particularly suitable for this treatment.
-
17M.3.hl.TZ2.26a.ii:
Outline how the alpha-radiation in TAT is directed to cancer cells.
-
17M.3.hl.TZ2.26b.i:
Identify the type of radiation emitted by these two radioisotopes.
-
17M.3.hl.TZ2.26b.ii:
State an equation for the one-step decay of yttrium-90.
-
17M.3.hl.TZ2.26b.iii:
The half-life of lutetium-177 is 6.75 days. Calculate the percentage remaining after 27 days.
-
17N.3.hl.TZ0.21a:
State a nuclear equation to show the decay of lutetium-177.
-
17N.3.hl.TZ0.21b:
The half-life of lutetium-177 is 6.73 days. Determine the percentage of a sample of lutetium-177 remaining after 14.0 days.
-
18M.3.hl.TZ1.19a:
Describe how ionizing radiation destroys cancer cells.
-
18M.3.hl.TZ1.19b:
Outline how Targeted Alpha Therapy (TAT) is used for treating cancers that have spread throughout the body.
-
18M.3.hl.TZ2.26a:
Phosphorous-32 undergoes beta decay. Formulate a balanced nuclear equation for this process.
-
18M.3.hl.TZ2.26b:
The half-life of phosphorus-32 is 14.3 days. Calculate the mass, in g, of 32P remaining after 57.2 days if the initial sample contains 2.63 × 10−8 mol. Use table 1 of the data booklet and Mr = 31.97 g mol−1.
-
18M.3.hl.TZ2.26c:
Explain the targeted alpha therapy (TAT) technique and why it is useful.
-
18N.3.hl.TZ0.22a:
Alpha particles are more damaging to human cells than any other nuclear radiation and yet they are used in targeted alpha therapy (TAT).
Explain how TAT is relatively safe to use in the treatment of dispersed cancers.
-
18N.3.hl.TZ0.22b.i:
Technetium-99m () has a half-life of 6.0 hours. Calculate the percentage of remaining in a sample of the radioisotope after two days.
-
18N.3.hl.TZ0.22b.ii:
Suggest why the percentage of technetium-99m remaining in the human body two days after injection will be lower than that calculated in (b)(i).
-
19M.3.hl.TZ1.24a(i):
Determine the percentage of technetium-99m remaining after 24.0 hours.
-
19M.3.hl.TZ1.24a(ii):
Technetium-99 decays further, emitting beta radiation. Formulate the equation for the decay of technetium-99.
-
19M.3.hl.TZ1.24b(ii):
Outline the disposal of LLW.
-
19M.3.hl.TZ1.24c:
Magnetic resonance imaging (MRI) is an application of NMR technology using radiowaves.
Suggest why MRI is much less dangerous than imaging techniques such as X-rays and radiotracers. Use section 3 of the data booklet.
-
19M.3.hl.TZ2.26a:
Evaluate the suitability of technetium-99m for this use.
-
19M.3.hl.TZ2.26b:
Calculate the percentage of technetium-99m remaining after 10.0 hours. Use section 1 of the data booklet.
- 19N.3.hl.TZ0.27a: State two common side effects of radiotherapy.
- 19N.3.hl.TZ0.27b: Explain why technetium-99m is the most common radioisotope used in nuclear medicine.
-
19N.3.hl.TZ0.27c:
25.0 μg of iodine-131, with a half-life of 8.00 days, was left to decay.
Calculate the mass of iodine-131, in μg, remaining after 32.0 days. Use section 1 of the data booklet.
-
20N.3.hl.TZ0.17a:
State the type of radiation technetium-99m emits.
- 20N.3.hl.TZ0.17b: Discuss the properties that make a radioisotope suitable for diagnosis.
-
20N.3.hl.TZ0.17d:
Technetium-99m has a half-life of hours. Calculate the amount of of technetium-99m remaining after hours.
D.9 Drug detection and analysis (HL only)
- 16N.3.hl.TZ0.29a: Suggest what may have led to these changes in acceptable concentrations.
-
16N.3.hl.TZ0.29b:
One class of performance-enhancing drugs is the anabolic steroids. Detection of these drugs in urine samples uses a combination of gas chromatography and mass spectrometry (GC/MS).
(i) Describe how gas chromatography enables the components of urine to be analysed.
(ii) The structures of two steroids, testosterone and nandrolone, are given below.
With reference to the molar masses of the two steroids, determine, with a reason, which can be identified from the mass spectrum below.
-
17M.3.hl.TZ1.25d:
Some mild analgesics contain a solid mixture of acidic aspirin and a non-acidic organic chemical of similar polarity to asprin.
Discuss how acid-base properties and the process of solvent extraction can be used to separate aspirin from the mixture.
-
17M.3.hl.TZ1.29d:
A breathalyser measures the blood alcohol content from a breath sample. Formulate half-equations for the reactions at the anode (negative electrode) and the cathode (positive electrode) in a fuel cell breathalyser.
- 17N.3.hl.TZ0.22a.i: Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
-
17N.3.hl.TZ0.22a.ii:
Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section 26 of the data booklet.
- 17N.3.hl.TZ0.27: Ethanol slows down the reaction time of a driver leading to traffic accidents. Explain how the...
-
18M.3.hl.TZ1.20a:
Hexane and propanone have vapour pressures of 17 kPa and 24 kPa respectively at 20 °C.
Calculate the vapour pressure, in kPa, at 20 °C of a mixture containing 60% hexane and 40% propanone by mole fraction, using Raoult’s law and assuming the mixture is ideal.
-
18M.3.hl.TZ1.20b:
Explain how hexane and propanone may be separated by fractional distillation.
-
18M.3.hl.TZ2.27a:
Fuel cells use an electrochemical process to determine the concentration of ethanol.
Formulate the overall equation for this process.
-
18M.3.hl.TZ2.27b:
Predict the chemical shifts and integration for each signal in the 1H NMR spectrum for ethanol using section 27 of the data booklet.
- 18N.3.hl.TZ0.21c: Explain the process of solvent extraction by which Taxol is isolated.
-
18N.3.hl.TZ0.23a:
State an analytical technique used to separate anabolic steroids from other compounds in an athlete’s urine or blood.
-
18N.3.hl.TZ0.23b:
Ethanol in breath can be detected by a redox reaction. Outline this method of detection. An equation is not required.
-
19M.3.hl.TZ1.22a(ii):
The resulting active metabolite of oseltamivir can be detected by mass spectrometry (MS) analysis.
Deduce the mass of the expected carboxylate ion.
Mr oseltamivir = 312
-
19M.3.hl.TZ2.27a:
Describe how a fuel cell breathalyser works.
-
19M.3.hl.TZ2.27b:
Alcohol levels in the breath can also be determined using IR spectroscopy.
Suggest, giving a reason, which bond’s absorbance is most useful for detecting ethanol in breath.
Bond:
Reason:
-
19N.3.hl.TZ0.24a:
Infrared (IR) spectroscopy is used to identify functional groups in organic compounds.
Deduce the wavenumber, in cm−1, of an absorption peak found in the IR spectrum of testosterone but not in that of cholesterol.
-
19N.3.hl.TZ0.24b:
Describe a technique for the detection of steroids in blood and urine.
-
19N.3.hl.TZ0.24c:
Explain how redox chemistry is used to measure the ethanol concentration in a breathalyser.
-
20N.3.hl.TZ0.15e:
Explain how IR spectroscopy can be used to distinguish aspirin from salicylic acid.
-
20N.3.hl.TZ0.18a(ii):
The vapour pressure of pure ethanal at is .
Calculate the vapour pressure of ethanal above the liquid mixture at .
- 20N.3.hl.TZ0.18b: Describe how this mixture is separated by fractional distillation.
