| Date | May 2018 | Marks available | 2 | Reference code | 18M.3.hl.TZ2.4 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Calculate | Question number | 4 | Adapted from | N/A |
Question
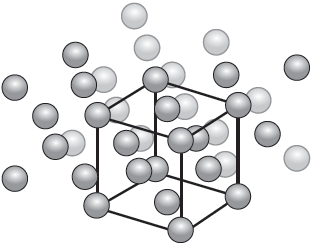
Vanadium forms a body centred cubic (BCC) crystal structure with an edge length of 303 pm, (303 × 10−12 m).
Deduce the number of atoms per unit cell in vanadium.
Calculate the expected first order diffraction pattern angle, in degrees, if x-rays of wavelength 150 pm are directed at a crystal of vanadium. Assume the edge length of the crystal to be the same as separation of layers of vanadium atoms found by x-ray diffraction. Use section 1 of the data booklet.
Calculate the average mass, in g, of a vanadium atom by using sections 2 and 6 of the data booklet.
Determine the volume, in cm3, of a vanadium unit cell.
Determine the density, in g cm−3, of vanadium by using your answers to (a)(i), (a)(iii) and (a)(iv).
Vanadium and other transition metals can interfere with cell metabolism.
State and explain one process, other than by creating free radicals, by which transition metals interfere with cell metabolism.
Vanadium(IV) ions can create free radicals by a Fenton reaction.
Deduce the equation for the reaction of V4+ with hydrogen peroxide.
Markscheme
2
[1 mark]
nλ = 2dsinθ
OR
θ = «» 14.3 «°»
Award [2] for correct final answer.
[2 marks]
m = « » 8.46 × 10–23 «g»
[1 mark]
«303 pm = 303 × 10–10 cm»
V = «(303 × 10–10)3 =» 2.78 × 10–23 «cm3 »
[1 mark]
«8.46 × 10–23 g × 2 =» 1.69 × 10–22 «g»
d = «» 6.08 «g cm–3»
Accept any value in the range 6.07–6.09 «g cm–3».
Award [2] for correct final answer.
[2 marks]
Any one of these alternatives:
ALTERNATIVE 1
disrupt enzyme binding sites
which can inhibit/over-stimulate enzymes
ALTERNATIVE 2
disrupt endocrine system
because they compete for active sites of enzymes/cellular receptors
ALTERNATIVE 3
form complexes/coordination compounds
which can bind to enzymes
ALTERNATIVE 4
act as oxidizing/reducing agents
OR
act as catalysts
which can initiate unwanted reactions
Accept “can undergo oxidation–reduction reactions” for M1 in Alternative 4.
[2 marks]
V4+(aq) + H2O2(aq) → V5+(aq) + OH–(aq) + •OH(aq)
Do not accept • on H.
Accept answer without •
[1 mark]