| Date | May 2019 | Marks available | 1 | Reference code | 19M.3.hl.TZ2.6 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | State | Question number | 6 | Adapted from | N/A |
Question
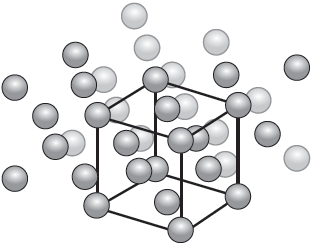
Calcium has a face-centred cubic (cubic close packing) arrangement of atoms.
State the number of atoms in the unit cell.
Determine the density of calcium, in g cm−3, using section 2 of the data booklet.
Ar = 40.08; metallic radius (r) = 1.97 × 10−10 m
Markscheme
« » 4 [✔]
a = « =» 5.572 × 10–10 «m»
OR
volume of unit cell = «(5.572 × 10–10 m)3 × 106 =» 1.73 × 10–22 «cm3» [✔]
mass of unit cell =« =» 2.66 × 10–22 «g» [✔]
density = « » 1.54 «g cm–3» [✔]
Note: Award [3] for correct final answer.
Examiners report
The number of atoms in the unit cell was correctly calculated by most of the candidates.
Majority of the candidates managed to get three marks in determining the density of the calcium.