| Date | May 2019 | Marks available | 2 | Reference code | 19M.3.sl.TZ1.7 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Calculate | Question number | 7 | Adapted from | N/A |
Question
Starch is a natural polymer of glucose.
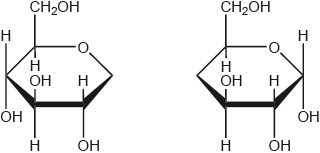
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
Explain how the inclusion of starch in plastics makes them biodegradable.
Markscheme
continuation bonds AND -O- attached to just one end AND both H-atoms on end carbons must be on the same side [✔]
Note: Square brackets not required.
Ignore “n” if given.
Mark may be awarded if a polymer is shown but with the repeating unit clearly identified.
Type of linkage:
glycosidic [✔]
Note: Accept “ether”.
(C6H10O5)n(s) + nH2O (l) → nC6H12O6 (aq) [✔]
Note: Accept “(n-1)H2O”.
Do not award mark if “n” not included.
q = «mcΔT = 975 g × 4.18 J g–1 K–1 × 15.0 K =» 61 100 «J» / 61.1 «kJ» [✔]
«heat per gram= =» 17.5 «kJ g–1» [✔]
Note: Award [2] for correct final answer.
Any two of:
carbohydrate grains swell/break plastic into smaller pieces [✔]
inclusion of carbohydrate makes the plastic more hydrophilic/water soluble [✔]
carbohydrates are broken down/hydrolysed/digested by bacteria/micro-organisms [✔]
plastic becomes more accessible to bacteria as holes/channels are created in it [✔]
«presence of» carbohydrate weakens intermolecular/London/dispersion forces between polymer chains in the plastic [✔]
Note: Accept “starch” for “carbohydrate” throughout. Do not accept carbohydrates are broken down/hydrolyzed.
Examiners report
Hardly any students were able to draw the required repeating unit, but in contrast almost all knew that the monomers were joined by a glycosidic linkage.
It was very unusual to find a candidate who could give a correct equation for starch hydrolysis.
Nearly half the students correctly calculated the enthalpy change and some of these went on to find the value in kJ g-1. The most common mistakes were to use the mass of starch rather than the mass of water and adding 273 to the temperature change.
Whilst many could quote from 7b, that starch undergoes hydrolysis, very few linked this to a biochemical mechanism. Other factors, relating to the reduction of intermolecular forces between the polymer chains were also rarely encountered.