| Date | November 2018 | Marks available | 3 | Reference code | 18N.3.hl.TZ0.5 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Determine | Question number | 5 | Adapted from | N/A |
Question
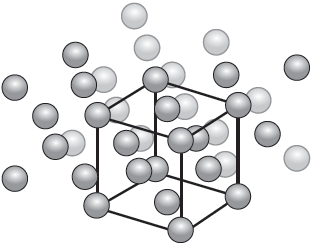
A representation of the unit cell of gold is shown.
State the name of the crystal structure of gold.
Calculate the number of atoms per unit cell of gold, showing your working.
The edge length of the gold unit cell is 4.08 × 10‒8 cm.
Determine the density of gold in g cm‒3, using sections 2 and 6 of the data booklet.
Markscheme
face-centred cube/fcc
OR
cubic close packed/ccp ✔
«atom per face» × 6 «faces per cube» × 3 «atoms» AND «atom per corner» × 8 «corners per cube» = 1 «atom» ✔
«atoms per unit cell = 3 + 1 =» 4 ✔
Award [1 max] for “4” without working shown.
«4 atoms per unit cell»
mass of 4 atoms «» 1.31 × 10–21 «g»
volume of unit cell «= (4.08 × 10‒8)3 cm3» = 6.79 × 10–23 «cm3»
density = «» = 1.93 × 101/19.3 «g cm‒3»
Award [3] for correct final answer.