| Date | November 2019 | Marks available | 3 | Reference code | 19N.3.hl.TZ0.10 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Describe | Question number | 10 | Adapted from | N/A |
Question
Aspartame is formed from the two amino acids aspartic acid (Asp) and phenylalanine (Phe).
Chromatography is used in the analysis of proteins in the food and pharmaceutical industry.
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
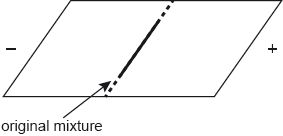
Describe, using another method, how a mixture of four amino acids, alanine, arginine, glutamic acid and glycine, could be separated when placed in a buffer solution of pH 6.0.
Suggest why alanine and glycine separate slightly at pH 6.5.
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
Markscheme
amide link (eg, CONH) ✔
correct order and structures of amino acids ✔
NOTE: Accept a skeletal formula or a full or condensed structural formula.
Accept zwitterion form of dipeptide.
Accept CO–NH but not CO–HN for amide link.
Any three of:
«gel» electrophoresis «technique»
OR
mixture «in buffer solution» placed on gel/paper ✔
voltage/potential «difference» applied ✔
amino acids move differently «depending on pH/isoelectric point» ✔
compare/measure distances travelled/Rf values ✔
NOTE: Accept “mixture placed on plate covered with polyacrylamide «gel» OR “mixture put in a gel «medium»”.
different sizes/molar masses/chain lengths «so move with different speeds» ✔
NOTE: Do not accept “different side-chains/R-groups/number of carbons”.
«6.0 = 4.83 + log »
«log= 1.17»
«[A−] : [HA] =» 14.8 : 1 ✔
NOTE: Accept “15:1”.
Do not accept 1:14.8.