DP Chemistry Questionbank

A: Materials
| Path: |
Description
[N/A]Directly related questions
-
16N.3.sl.TZ0.4e:
Deduce the charge on the indium ion and the formula of indium sulfate.
- 16N.3.sl.TZ0.7b: Explain the effect of increasing the temperature of a nematic liquid crystal on its directional...
-
16N.3.hl.TZ0.10b:
Adsorption and chelation are two methods of removing heavy metal ion pollution from the environment.
(i) Describe the process of adsorption.
(ii) Deduce the structure of the complex ion formed by the reaction of three H2N−CH2−CH2−NH2 chelating molecules with a mercury(II) ion.
-
16N.3.hl.TZ0.8a:
(i) The diagram below shows the diffraction of two X-ray beams, y and z of wavelength λ, shining on a chromium crystal whose planes are a distance d nm apart.
Deduce the extra distance travelled by the second beam, z, compared to the first one, y.
(ii) State the Bragg’s condition for the observed diffraction to be at its strongest (constructive interference).
- 16N.3.hl.TZ0.9b: Outline one difference between type 1 and type 2 superconductors.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
- 16N.3.hl.TZ0.10a: Compare and contrast the Fenton and Haber–Weiss reaction mechanisms.
-
16N.3.sl.TZ0.6a:
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
-
16N.3.sl.TZ0.5b:
Nanocatalysts play an essential role in the manufacture of industrial chemicals.
(i) Describe the high pressure carbon monoxide (HIPCO) method for the production of carbon nanotubes.
(ii) Outline one benefit of using nanocatalysts compared to traditional catalysts in industry.
- 16N.3.hl.TZ0.9a: Describe the Meissner effect.
- 16N.3.sl.TZ0.3a: Magnesium oxide, MgO, and silicon carbide, SiC, are examples of ceramic materials. State the name...
-
16N.3.sl.TZ0.4a:
Calculate the charge, in coulombs, passed during the electrolysis.
-
16N.3.sl.TZ0.4b:
Calculate the amount, in mol, of electrons passed using section 2 of the data booklet.
-
16N.3.sl.TZ0.4c:
Calculate the mass of indium deposited by one mole of electrons.
-
16N.3.sl.TZ0.4d:
Calculate the number of moles of electrons required to deposit one mole of indium. Relative atomic mass of indium, Ar=114.82.
- 16N.3.sl.TZ0.5a: Explain, with reference to their structure, the great selectivity of zeolites as catalysts.
- 16N.3.sl.TZ0.7a: Outline how a lyotropic liquid crystal differs from a thermotropic liquid crystal.
-
16N.3.hl.TZ0.6d:
Fermentation of sugars from corn starch produces propane-1,3-diol, which can be polymerized with benzene-1,4-dicarboxylic acid to produce the PTT polymer (polytrimethylene terephthalate).
(i) Draw the molecular structure of each monomer.
(ii) Deduce the name of the linkage formed on polymerization between the two monomers and the name of the inorganic product.
- 16N.3.sl.TZ0.3b: Predict the predominant type of bonding for a binary compound AB in which the electronegativity...
-
16N.3.sl.TZ0.6b:
Deduce the percentage atom economy for polymerization of 2-methylpropene.
-
16N.3.hl.TZ0.8b:
(i) The mass of one unit cell of chromium metal is 17.28 × 10−23 g. Calculate the number of unit cells in one mole of chromium. Ar(Cr) = 52.00.
(ii) Deduce the number of atoms of chromium per unit cell.
- 20N.3.sl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.sl.TZ0.3c: Explain why phthalates are replaced by other plasticizers in the production of plastics.
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
- 20N.3.sl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
- 20N.3.sl.TZ0.4d: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
- 20N.3.sl.TZ0.4a: Explain these properties of carbon nanotubes.
-
20N.3.sl.TZ0.4b(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020.
Write the half-equations for the reactions occurring in this electrolysis.
-
20N.3.sl.TZ0.3a:
Outline the two distinct phases of this composite.
-
20N.3.sl.TZ0.4b(iv):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
- 20N.3.sl.TZ0.4c: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
-
20N.3.sl.TZ0.4b(iii):
Calculate the theoretical mass of magnesium obtained if a current of is used for hours. Use charge and section 2 of the data booklet
- 20N.3.hl.TZ0.3b(iii): Explain why phthalates are replaced by other plasticizers in the production of plastics.
- 20N.3.hl.TZ0.4d: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
-
20N.3.hl.TZ0.3a:
Outline the two distinct phases of this composite.
- 20N.3.hl.TZ0.4b(i): CNT can act as Type 2 superconductors. Outline why Type 2 superconductors are generally more...
- 20N.3.hl.TZ0.4a: Explain these properties of carbon nanotubes.
-
20N.3.hl.TZ0.5a:
Precipitation is one method used to treat waste water.
Phosphates, , in waste water can be removed by precipitation with magnesium ions. of magnesium phosphate is .
Calculate the maximum solubility of phosphate ions in a solution containing magnesium ions.
- 20N.3.hl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.hl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
-
20N.3.hl.TZ0.4c(iii):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
-
20N.3.hl.TZ0.4b(ii):
Explain the role of electrons in superconducting materials in terms of the Bardeen–Cooper–Schrieffer (BCS) theory.
- 20N.3.hl.TZ0.3c: Classify PVC and polyethene terephthalate, PET, as addition or condensation polymers and deduce...
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
-
20N.3.hl.TZ0.5b:
Precipitation is one method used to treat waste water.
Zinc, cadmium, nickel, and lead are metal ions which can be removed by precipitation. Explain why waste water is adjusted to a pH of 9−10 to remove these ions by referring to section 32 of the data booklet.
-
20N.3.hl.TZ0.4c(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020
Calculate the theoretical mass of magnesium obtained if a current of 3.00 A is used for hours. Use charge :(Q) = current (I) × time (t) and section 2 of the data booklet.
- 20N.3.hl.TZ0.4e: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
-
17M.3.sl.TZ1.6a:
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
-
17M.3.sl.TZ1.6b:
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
-
17M.3.sl.TZ1.7a:
State why lanthanum cannot be produced by reducing its oxide with carbon.
-
17M.3.sl.TZ1.7b:
Calculate the current (I), in A, required to produce 1.00 kg of lanthanum metal per hour. Use the formula and sections 2 and 6 of the data booklet.
-
17M.3.sl.TZ1.8a:
State the major advantage that nanoparticles have in these applications.
-
17M.3.sl.TZ1.8b:
Suggest why nanoparticles need to be handled with care.
-
17M.3.sl.TZ1.9a:
Catalysts reduce the activation energy. Outline how homogeneous catalysts are involved in the reaction mechanism.
-
17M.3.sl.TZ1.9b:
Suggest why it is important to know how catalysts function.
-
17M.3.sl.TZ1.9c:
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.sl.TZ1.10a:
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
-
17M.3.sl.TZ1.10b.i:
Describe the difference in their structures.
-
17M.3.sl.TZ1.10b.ii:
Explain why the difference in their structures affects their melting points.
-
17M.3.hl.TZ1.8a:
Lanthanum has a hexagonal close packed (hcp) crystal structure. State the coordination number of each lanthanum atom.
-
17M.3.hl.TZ1.8b:
Lanthanum becomes superconducting below 5 K. Explain, in terms of Bardeen–Cooper–Schrieffer (BCS) theory, how superconductivity occurs.
-
17M.3.hl.TZ1.8c:
Outline why superconductivity only occurs at low temperatures.
-
17M.3.hl.TZ1.9a:
Deduce the repeating unit of the polymer and the other product of the reaction.
-
17M.3.hl.TZ1.9b:
State the class of polymer to which PETE belongs.
-
17M.3.hl.TZ1.10a:
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.hl.TZ1.10b:
Hydrogen sulfide could be used to remove antimony(III) ions from a solution.
Determine the concentration of antimony(III) ions that would be required to precipitate antimony(III) sulfide in a solution saturated with hydrogen sulfide.
[S2−] in water saturated with hydrogen sulfide = 1.0 × 10−14 mol dm−3
Ksp (Sb2S3) = 1.6 × 10−93
-
17M.3.hl.TZ1.10c:
Identify a ligand that could be used to chelate antimony(III) ions in solution.
-
17M.3.sl.TZ2.3a:
State the two distinct phases of a composite.
-
17M.3.sl.TZ2.3b:
Identify the methods of assembling nanocomposites by completing the table.
-
17M.3.sl.TZ2.3c.i:
Explain how the structure of plasticizers enables them to soften PVC.
-
17M.3.sl.TZ2.3c.ii:
Suggest a reason why nanoparticles can better anchor plasticizers in the polymer.
-
17M.3.sl.TZ2.5a:
In a catalytic converter, carbon monoxide is converted to carbon dioxide. Outline the process for this conversion referring to the metal used.
-
17M.3.sl.TZ2.5b.i:
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
-
17M.3.sl.TZ2.5c:
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
-
17M.3.sl.TZ2.6a:
Two important properties of a liquid crystal molecule are being a polar molecule and having a long alkyl chain. Explain why these are essential components of a liquid crystal molecule.
-
17M.3.sl.TZ2.6b:
Metal impurities during the production of LCoS can be analysed using ICP-MS. Each metal has a detection limit below which the uncertainty of data is too high to be valid. Suggest one factor which might influence a detection limit in ICP-MS/ICP-OES.
-
17M.3.hl.TZ2.3c:
Estimate the atom economy of this first step.
-
17M.3.hl.TZ2.3c.ii:
Suggest, giving one reason, whether this is an addition or condensation reaction.
-
17M.3.hl.TZ2.4a:
Identify the other product formed.
-
17M.3.hl.TZ2.4b:
Explain why EDTA, a chelating agent, is more effective in removing heavy metal ions from solution than monodentate ligands.
-
17M.3.hl.TZ2.5b.iii:
Nickel(II) ions are least soluble at pH 10.5. Calculate the molar solubility of nickel(II) hydroxide at this pH. KspNi(OH)2 = 5.48 × 10–16.
-
17M.3.hl.TZ2.5c.i:
Rhodium is paramagnetic with an electron configuration of [Kr] 5s14d8.
Explain, in terms of electron spin pairing, why paramagnetic substances are attracted to a magnetic field and diamagnetic substances are not.
-
17M.3.hl.TZ2.5c.ii:
Rhodium is a type 1 superconductor.
Sketch graphs of resistance against temperature for a conductor and superconductor.
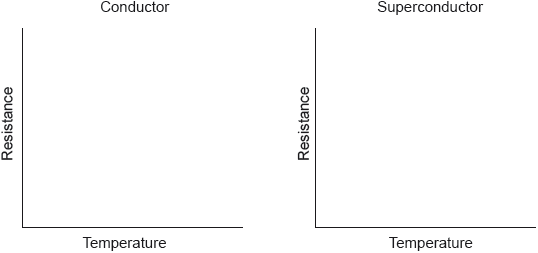
-
17M.3.hl.TZ2.5c.iii:
Contrast type 1 and type 2 superconductors by referring to three differences between them.
-
17N.3.sl.TZ0.4a:
Outline the composition of an alloy and a composite.
-
17N.3.sl.TZ0.6a:
State equations for the formation of iron nanoparticles and carbon atoms from Fe(CO)5 in the HIPCO process.
- 17N.3.hl.TZ0.7b: Describe how the monomers of addition polymers and of condensation polymers differ.
-
17N.3.hl.TZ0.9b:
The solubility product, Ksp , of cadmium sulfide, CdS, is 8.0 × 10–27. Determine the concentration of cadmium ions in 1.0 dm3 of a saturated solution of cadmium sulfide to which 0.10 mol of solid sodium sulfide has been added, stating any assumption you make.
-
17N.3.hl.TZ0.8a:
Calculate the total number of cobalt atoms within its unit cell.
-
17N.3.sl.TZ0.7b.ii:
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
-
17N.3.hl.TZ0.6b:
Explain why Type 2 superconductors are generally more useful than Type 1.
-
17N.3.hl.TZ0.7c:
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
-
17N.3.hl.TZ0.8b.i:
The atomic radius, r, of cobalt is 1.18 × 10–8 cm. Determine the edge length, in cm, of the unit cell, a, using the second diagram.
-
17N.3.hl.TZ0.8b.ii:
Determine a value for the density of cobalt, in g cm–3, using data from sections 2 and 6 of the data booklet and your answers from (a) and (b) (i).
If you did not obtain an answer to (b) (i), use 3.00 × 10–8 cm but this is not the correct answer.
- 17N.3.hl.TZ0.9a: State the name of one method, other than precipitation, of removing heavy metal ions from...
- 17N.3.sl.TZ0.4b.i: Outline why an alloy is usually harder than its components by referring to its structure.
- 17N.3.sl.TZ0.4b.ii: At present, composite fillings are more expensive than amalgam fillings. Suggest why a patient...
-
17N.3.sl.TZ0.4c:
Explain how Inductively Coupled Plasma (ICP) Spectroscopy could be used to determine the concentration of mercury in a sample of dental filling.
-
17N.3.sl.TZ0.5:
Catalysts can take many forms and are used in many industrial processes.
Suggest two reasons why it might be worth using a more expensive catalyst to increase the rate of a reaction.
-
17N.3.sl.TZ0.7a:
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
-
17N.3.sl.TZ0.7c:
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
- 17N.3.sl.TZ0.6c: Discuss one possible risk associated with the use of nanotechnology.
- 17N.3.sl.TZ0.6b: Outline why the iron nanoparticle catalysts produced by the HIPCO process are more efficient than...
-
17N.3.sl.TZ0.7b.i:
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
-
18M.3.hl.TZ1.4c.i:
Draw the structure of the monomer from which nylon-6 is produced by a condensation reaction.
-
18M.3.hl.TZ2.4a.i:
Deduce the number of atoms per unit cell in vanadium.
-
18M.3.hl.TZ2.4b.i:
Vanadium and other transition metals can interfere with cell metabolism.
State and explain one process, other than by creating free radicals, by which transition metals interfere with cell metabolism.
-
18M.3.hl.TZ1.4c.ii:
Deduce, giving a reason, whether the atom economy of a condensation polymerization, such as this, would be greater or less than an addition polymerization, such as the formation of HDPE.
-
18M.3.hl.TZ1.5b:
The diagram illustrates the crystal structure of aluminium metal with the unit cell indicated. Outline the significance of the unit cell.
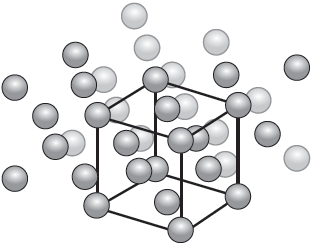
-
18M.3.hl.TZ1.5e:
The concentration of aluminium in drinking water can be reduced by precipitating aluminium hydroxide. Calculate the maximum concentration of aluminium ions in water of pH 7 at 298 K. Solubility product of aluminium hydroxide = 3.3 × 10−34 at 298 K.
-
18M.3.hl.TZ1.5c:
When X-rays of wavelength 0.154 nm are directed at a crystal of aluminium, the first order diffraction pattern is observed at 18°. Determine the separation of layers of aluminium atoms in the crystal, in m, using section 1 of the data booklet.
-
18M.3.hl.TZ2.5c.i:
Distinguish between the manufacture of polyester and polyethene.
-
18M.3.hl.TZ1.5d.i:
Deduce what the shape of the graph indicates about aluminium.
-
18M.3.hl.TZ2.4a.iii:
Calculate the average mass, in g, of a vanadium atom by using sections 2 and 6 of the data booklet.
-
18M.3.hl.TZ2.6b:
MWCNT are very small in size and can greatly increase switching speeds in a liquid crystal allowing the liquid crystal to change orientation quickly.
Discuss two other properties a substance should have to be suitable for use in liquid crystal displays.
-
18M.3.hl.TZ2.4a.ii:
Calculate the expected first order diffraction pattern angle, in degrees, if x-rays of wavelength 150 pm are directed at a crystal of vanadium. Assume the edge length of the crystal to be the same as separation of layers of vanadium atoms found by x-ray diffraction. Use section 1 of the data booklet.
-
18M.3.hl.TZ2.4a.iv:
Determine the volume, in cm3, of a vanadium unit cell.
-
18M.3.hl.TZ1.5d.ii:
Outline why the resistance of aluminium increases above 1.2 K.
-
18M.3.hl.TZ2.4a.v:
Determine the density, in g cm−3, of vanadium by using your answers to (a)(i), (a)(iii) and (a)(iv).
-
18M.3.hl.TZ2.4b.ii:
Vanadium(IV) ions can create free radicals by a Fenton reaction.
Deduce the equation for the reaction of V4+ with hydrogen peroxide.
-
18M.3.sl.TZ1.3a:
Discuss, in terms of its structure, why an aluminium saucepan is impermeable to water.
-
18M.3.sl.TZ1.3b.i:
State the name given to a material composed of two distinct solid phases.
-
18M.3.sl.TZ1.3b.ii:
State one physical property of HDPE that will be affected by the incorporation of carbon nanotubes.
-
18M.3.sl.TZ1.3b.iii:
Describe how carbon nanotubes are produced by chemical vapour deposition (CVD).
-
18M.3.sl.TZ1.3b.iv:
State the property of carbon nanotubes that enables them to form a nematic liquid crystal phase.
-
18M.3.sl.TZ1.4a:
Both of these are thermoplastic polymers. Outline what this term means.
-
18M.3.sl.TZ1.4b.i:
Compare and contrast the structures of HDPE and LDPE.
-
18M.3.sl.TZ1.4b.ii:
State one way in which a physical property of HDPE, other than density, differs from that of LDPE as a result of this structural difference.
-
18M.3.sl.TZ1.4c.i:
The production of HDPE involves the use of homogeneous catalysts. Outline how homogeneous catalysts reduce the activation energy of reactions.
-
18M.3.sl.TZ1.4c.ii:
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
-
18M.3.sl.TZ1.4d:
Suggest two of the major obstacles, other than collection and economic factors, which have to be overcome in plastic recycling.
-
18M.3.sl.TZ1.4e:
Suggest why there are so many different ways in which plastics can be classified. HDPE can, for example, be categorized thermoplastic, an addition polymer, having Resin Identification Code (RIC) 2, etc.
-
18M.3.sl.TZ1.5:
Aluminium is produced by the electrolysis of a molten electrolyte containing bauxite.
Determine the mass, in g, of aluminium produced by the passage of a charge of 1.296 × 1013 C. Use sections 2 and 6 of the data booklet.
-
18M.3.sl.TZ2.3a:
ICP-OES/MS can be used to analyse alloys and composites. Distinguish between alloys and composites.
-
18M.3.sl.TZ2.3b:
ICP-MS is a reference mode for analysis. The following correlation graphs between ICP-OES and ICP-MS were produced for yttrium and nickel.
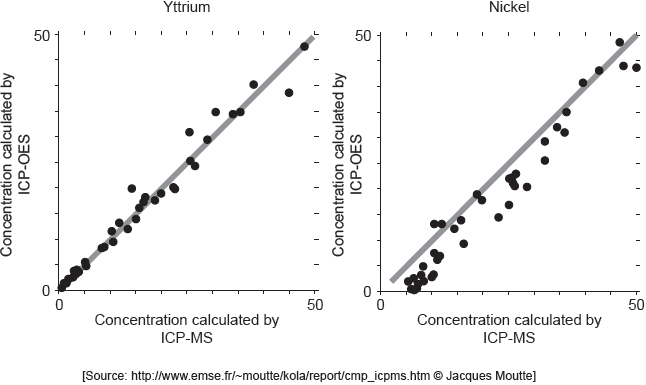
Each y-axis shows concentrations calculated by ICP-OES; each x-axis shows concentrations for the same sample as found by ICP-MS.
The line in each graph is y = x.
Discuss the effectiveness of ICP-OES for yttrium and nickel.
-
18M.3.sl.TZ2.3c.i:
Identify the purpose of each graph.
-
18M.3.sl.TZ2.3c.ii:
Calculate, to four significant figures, the concentration, in μg kg−1, of vanadium in oil giving a signal intensity of 14 950.
-
18M.3.sl.TZ2.3c.iii:
Vanadium(V) oxide is used as the catalyst in the conversion of sulfur dioxide to sulfur trioxide.
SO2(g) + V2O5(s) → SO3(g) + 2VO2(s)
O2(g) + 2VO2(s) → V2O5(s)
Outline how vanadium(V) oxide acts as a catalyst.
-
18M.3.sl.TZ2.4a:
Sketch four repeating units of the polymer to show atactic and isotactic polypropene.
-
18M.3.sl.TZ2.4b.i:
State the chemical reason why plastics do not degrade easily.
-
18M.3.sl.TZ2.5a:
State the source of carbon for MWCNT produced by arc discharge and by CVD.
-
18M.3.sl.TZ2.4b.ii:
Compare two ways in which recycling differs from reusing plastics.
-
18M.3.sl.TZ2.5b:
Discuss three properties a substance should have to be suitable for use in liquid crystal displays.
-
18M.3.sl.TZ2.4c:
Civilizations are often characterized by the materials they use.
Suggest an advantage polymers have over materials from the iron age.
-
18N.3.hl.TZ0.2b:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
-
18N.3.sl.TZ0.2b.ii:
In an incomplete combustion of the polyvinyl chloride, PVC, it was found that hydrogen chloride, carbon monoxide, carbon dioxide, and water vapour were released.
Formulate an equation for this reaction using the formula of the PVC repeating unit.
-
18N.3.sl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
-
18N.3.sl.TZ0.3b:
An unknown antacid sample has a lead ion concentration of 0.50 μg dm‒3.
Calculate the concentration of lead ions in the sample in mol dm‒3.
- 18N.3.sl.TZ0.2c.i: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
-
18N.3.hl.TZ0.3d.ii:
An aqueous lead(II) ion reacts with three ethane-1,2-diamine molecules to form an octahedral chelate ion.
Outline why the chelate ion is more stable than the reactants.
-
18N.3.sl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
-
18N.3.sl.TZ0.4c:
Arc discharge, consisting of two inert metal electrodes in a liquid solvent, is one method of producing carbon nanotubes (CNTs).
Predict, giving a reason, the electrode at which the solvent cyclohexane, C6H12, will decompose to form CNTs.
-
18N.3.hl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
- 18N.3.hl.TZ0.2d.i: State the names of the two terminal functional groups in X.
- 18N.3.sl.TZ0.4a: Outline two observations that he could have made.
-
18N.3.hl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
-
18N.3.sl.TZ0.2d:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
- 18N.3.hl.TZ0.2c: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
- 18N.3.sl.TZ0.3a: State the type of particle present in the plasma formed.
-
18N.3.sl.TZ0.2b.i:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
- 18N.3.sl.TZ0.2c.ii: Identify another advantage of using a zeolite instead of concentrated sulfuric acid.
- 18N.3.hl.TZ0.2d.ii: Deduce the repeating unit of the polymer of X.
-
18N.3.hl.TZ0.3b.i:
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
18N.3.hl.TZ0.3b.ii:
Lead ions are toxic and can be precipitated using hydroxide ions.
Pb2+ (aq) + 2OH‒ (aq) Pb(OH)2 (s)
Sufficient sodium hydroxide solid is added to the antacid sample to produce a 1.0 × 10‒2 mol dm‒3 hydroxide ion solution at 298 K.
Deduce if a precipitate will be formed, using section 32 of the data booklet.
If you did not calculate the concentration of lead ions in (b)(i), use the value of 2.4 × 10−4 mol dm‒3, but this is not the correct value.
- 18N.3.sl.TZ0.4b: The structure of biphenyl nitrile is shown. Describe, giving a reason, a feature of the...
- 18N.3.hl.TZ0.3a: State the type of particle present in the plasma formed.
-
18N.3.hl.TZ0.5a.ii:
Calculate the number of atoms per unit cell of gold, showing your working.
- 18N.3.hl.TZ0.3d.i: State one feature of a chelating agent.
- 18N.3.hl.TZ0.5a.i: State the name of the crystal structure of gold.
-
18N.3.hl.TZ0.5b:
The edge length of the gold unit cell is 4.08 × 10‒8 cm.
Determine the density of gold in g cm‒3, using sections 2 and 6 of the data booklet.
-
18N.3.hl.TZ0.2d.iii:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
-
19M.3.hl.TZ1.4e:
State one factor considered when making green chemistry polymers.
-
19M.3.hl.TZ1.3e:
Lithium forms a crystalline lattice with the unit cell structure shown below.
X-ray diffraction shows that the length of the edge of the unit cell is 3.51 × 10−8 cm.
Determine the density of lithium, in g cm−3, using sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.hl.TZ1.4c:
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
-
19M.3.hl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.hl.TZ1.4d:
Classify polybutadiene as either an addition or condensation polymer, giving a reason.
-
19M.3.hl.TZ1.3b(iii):
Suggest a better method.
-
19M.3.hl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.hl.TZ1.3a:
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.hl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.hl.TZ1.3d(i):
Lithium has shown some superconductive properties when doped into graphene or when under high pressure. Under high pressure, however, the Meissner effect is absent.
Describe the Meissner effect.
-
19M.3.hl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.hl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.hl.TZ1.7b:
State the number of coordinate covalent bonds EDTA forms with Ni2+.
-
19M.3.hl.TZ1.3b(ii):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.hl.TZ1.3d(ii):
At very low temperatures, lithium atoms enhance the phonon binding of electrons in graphene suggesting the formation of Cooper pairs.
Explain how Cooper pairs are formed.
-
19M.3.hl.TZ1.5a:
Label with an asterisk, *, the chiral carbon atom.
-
19M.3.hl.TZ1.7a:
Explain how entropy affects this equilibrium.
-
19M.3.hl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19M.3.hl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase.
Shape of molecules:
Distribution:
-
19M.3.hl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.hl.TZ2.5e:
Outline, giving a reason, how addition and condensation polymerization compare with regard to green chemistry.
-
19M.3.hl.TZ2.5f:
Draw the full structural formula of the organic functional group formed during the polymerization of the two reactants below.
-
19M.3.hl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.hl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19M.3.hl.TZ2.5d:
Suggest why the addition of plasticizers is controversial.
-
19M.3.hl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48 250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19M.3.hl.TZ2.7a:
State what is meant by a superconductor.
-
19M.3.hl.TZ2.6a:
State the number of atoms in the unit cell.
-
19M.3.hl.TZ2.18b:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.hl.TZ2.5c:
Explain how plasticizers affect the properties of plastics.
-
19M.3.hl.TZ2.8a:
Outline why heavy metals are toxic.
-
19M.3.hl.TZ2.8b:
Determine the maximum concentration of lead(II) ions at 298 K in a solution in which the concentration of carbonate ions is maintained at 1.10 × 10−4 mol dm−3. Use section 32 of the data booklet.
-
19M.3.hl.TZ2.6b:
Determine the density of calcium, in g cm−3, using section 2 of the data booklet.
Ar = 40.08; metallic radius (r) = 1.97 × 10−10 m
-
19M.3.hl.TZ2.8c:
State a method, other than precipitation, of removing heavy metal ions from solution.
-
19M.3.sl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.sl.TZ1.4c(i):
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.sl.TZ1.3b(ii):
Suggest a better method.
-
19M.3.sl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.sl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.sl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.sl.TZ1.4c(ii):
The trace quantities of dioxins from tyre fires are rarely inhaled and instead settle on the ground.
Describe why this is a health concern.
-
19M.3.sl.TZ1.3a(ii):
Explain why lithium is paramagnetic while lithium hydride is diamagnetic by referring to electron configurations.
-
19M.3.sl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.sl.TZ1.5a:
State the name of the functional group which allows the molecule to be responsive to applied electric fields.
-
19M.3.sl.TZ1.3a(i):
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.sl.TZ1.3b(i):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.sl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.sl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.sl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19M.3.sl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.sl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19M.3.sl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.sl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19M.3.sl.TZ2.5c:
Identify a hazardous product of the incineration of polychloroethene.
-
19M.3.sl.TZ2.5d:
Explain how plasticizers affect the properties of plastics.
-
19M.3.sl.TZ2.5e:
Suggest why the addition of plasticizers is controversial.
-
19M.3.sl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase and the effect that an electric field has on it.
Shape of molecules:Distribution:
Effect of electric field:
- 19N.3.hl.TZ0.5c: Discuss why the recycling of plastics is an energy intensive process.
- 19N.3.sl.TZ0.4c: Polypropene is a thermoplastic. Outline what is meant by thermoplastic.
-
19N.3.sl.TZ0.5a:
Discuss why different methods of reduction are needed to extract metals.
- 19N.3.sl.TZ0.6b: State how liquid crystals are affected by an electric field.
- 19N.3.sl.TZ0.4d: Discuss why the recycling of plastics is an energy intensive process.
- 19N.3.hl.TZ0.4b: State two differences between Type I and Type II superconductors.
- 19N.3.sl.TZ0.6a: Describe the arrangement of soap molecules in the nematic liquid crystal phase.
-
19N.3.sl.TZ0.4a:
Draw a section of an isotactic polypropene polymer chain containing four repeating units.
- 19N.3.sl.TZ0.3b: Distinguish between heterogeneous and homogeneous catalysts, giving one difference.
- 19N.3.sl.TZ0.4b: Predict, with a reason, whether isotactic or atactic polypropene has the higher melting point.
- 19N.3.hl.TZ0.4a(ii): Suggest why the resistance of metals increases with temperature.
-
19N.3.sl.TZ0.5b(i):
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
-
19N.3.sl.TZ0.3a:
Describe how a heterogeneous catalyst provides an alternative pathway for a reaction.
- 19N.3.hl.TZ0.4a(i): Outline how resistance to electric currents occurs in metals.
-
19N.3.hl.TZ0.5a:
Draw the structure of the monomers of Kevlar® if the by-product of the condensation polymerization is hydrogen chloride.
-
19N.3.sl.TZ0.3c:
Nanotubes are used to support the active material in nanocatalysts.
Explain why oxygen cannot be used for the chemical vapour deposition (CVD) preparation of carbon nanotubes.
-
19N.3.hl.TZ0.7:
X-ray crystallography of a metal crystal produces a diffraction pattern of bright spots.
Using X-rays of wavelength 1.54 × 10−10 m, the first bright spots were produced at an angle θ of 22.3° from the centre.
Calculate the separation between planes of atoms in the lattice, in meters, using section 1 of the data booklet.
-
19N.3.hl.TZ0.8:
1.40 × 10−3 g of NaOH (s) are dissolved in 250.0 cm3 of 1.00 × 10−11 mol dm−3 Pb(OH)2 (aq) solution.
Determine the change in lead ion concentration in the solution, using section 32 of the data booklet.
-
19N.3.sl.TZ0.5b(ii):
Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
- 19N.3.hl.TZ0.5b: State and explain why plasticizers are added to polymers.
Sub sections and their related questions
A.1 Materials science introduction
- 16N.3.sl.TZ0.3a: Magnesium oxide, MgO, and silicon carbide, SiC, are examples of ceramic materials. State the name...
- 16N.3.sl.TZ0.3b: Predict the predominant type of bonding for a binary compound AB in which the electronegativity...
-
17M.3.sl.TZ1.6a:
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
-
17M.3.sl.TZ1.6b:
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
-
17M.3.sl.TZ2.3a:
State the two distinct phases of a composite.
-
17M.3.sl.TZ2.3b:
Identify the methods of assembling nanocomposites by completing the table.
-
17N.3.sl.TZ0.4a:
Outline the composition of an alloy and a composite.
- 17N.3.sl.TZ0.4b.ii: At present, composite fillings are more expensive than amalgam fillings. Suggest why a patient...
-
18M.3.sl.TZ1.3a:
Discuss, in terms of its structure, why an aluminium saucepan is impermeable to water.
-
18M.3.sl.TZ1.3b.i:
State the name given to a material composed of two distinct solid phases.
-
18M.3.sl.TZ1.4e:
Suggest why there are so many different ways in which plastics can be classified. HDPE can, for example, be categorized thermoplastic, an addition polymer, having Resin Identification Code (RIC) 2, etc.
-
18M.3.sl.TZ2.3a:
ICP-OES/MS can be used to analyse alloys and composites. Distinguish between alloys and composites.
-
18M.3.sl.TZ2.4c:
Civilizations are often characterized by the materials they use.
Suggest an advantage polymers have over materials from the iron age.
-
18N.3.sl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
-
18N.3.hl.TZ0.2a:
Outline why this type of classification is not entirely satisfactory by using magnesium diboride, MgB2, as an example. Refer to sections 8 and 29 of the data booklet.
-
19M.3.hl.TZ1.3a:
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.hl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19M.3.sl.TZ1.3a(i):
Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
-
19M.3.sl.TZ2.4d:
Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
-
19N.3.sl.TZ0.5b(i):
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
-
20N.3.sl.TZ0.3a:
Outline the two distinct phases of this composite.
-
20N.3.hl.TZ0.3a:
Outline the two distinct phases of this composite.
A.2 Metals and inductively coupled plasma (ICP) spectroscopy
-
16N.3.sl.TZ0.4a:
Calculate the charge, in coulombs, passed during the electrolysis.
-
16N.3.sl.TZ0.4b:
Calculate the amount, in mol, of electrons passed using section 2 of the data booklet.
-
16N.3.sl.TZ0.4c:
Calculate the mass of indium deposited by one mole of electrons.
-
16N.3.sl.TZ0.4d:
Calculate the number of moles of electrons required to deposit one mole of indium. Relative atomic mass of indium, Ar=114.82.
-
16N.3.sl.TZ0.4e:
Deduce the charge on the indium ion and the formula of indium sulfate.
-
17M.3.sl.TZ1.7a:
State why lanthanum cannot be produced by reducing its oxide with carbon.
-
17M.3.sl.TZ1.7b:
Calculate the current (I), in A, required to produce 1.00 kg of lanthanum metal per hour. Use the formula and sections 2 and 6 of the data booklet.
-
17M.3.sl.TZ1.9c:
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.hl.TZ1.10a:
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.sl.TZ2.5b.i:
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
-
17M.3.sl.TZ2.5c:
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
-
17M.3.sl.TZ2.6b:
Metal impurities during the production of LCoS can be analysed using ICP-MS. Each metal has a detection limit below which the uncertainty of data is too high to be valid. Suggest one factor which might influence a detection limit in ICP-MS/ICP-OES.
-
17M.3.hl.TZ2.5c.i:
Rhodium is paramagnetic with an electron configuration of [Kr] 5s14d8.
Explain, in terms of electron spin pairing, why paramagnetic substances are attracted to a magnetic field and diamagnetic substances are not.
- 17N.3.sl.TZ0.4b.i: Outline why an alloy is usually harder than its components by referring to its structure.
-
17N.3.sl.TZ0.4c:
Explain how Inductively Coupled Plasma (ICP) Spectroscopy could be used to determine the concentration of mercury in a sample of dental filling.
-
18M.3.sl.TZ1.4c.ii:
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
-
18M.3.sl.TZ1.5:
Aluminium is produced by the electrolysis of a molten electrolyte containing bauxite.
Determine the mass, in g, of aluminium produced by the passage of a charge of 1.296 × 1013 C. Use sections 2 and 6 of the data booklet.
-
18M.3.sl.TZ2.3b:
ICP-MS is a reference mode for analysis. The following correlation graphs between ICP-OES and ICP-MS were produced for yttrium and nickel.

Each y-axis shows concentrations calculated by ICP-OES; each x-axis shows concentrations for the same sample as found by ICP-MS.
The line in each graph is y = x.
Discuss the effectiveness of ICP-OES for yttrium and nickel.
-
18M.3.sl.TZ2.3c.i:
Identify the purpose of each graph.
-
18M.3.sl.TZ2.3c.ii:
Calculate, to four significant figures, the concentration, in μg kg−1, of vanadium in oil giving a signal intensity of 14 950.
- 18N.3.sl.TZ0.3a: State the type of particle present in the plasma formed.
-
18N.3.sl.TZ0.3b:
An unknown antacid sample has a lead ion concentration of 0.50 μg dm‒3.
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
18N.3.sl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
- 18N.3.hl.TZ0.3a: State the type of particle present in the plasma formed.
-
18N.3.hl.TZ0.3b.i:
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
18N.3.hl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
-
19M.3.hl.TZ1.3b(ii):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.hl.TZ1.3b(iii):
Suggest a better method.
-
19M.3.hl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.hl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48 250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.sl.TZ1.3a(ii):
Explain why lithium is paramagnetic while lithium hydride is diamagnetic by referring to electron configurations.
-
19M.3.sl.TZ1.3b(i):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.sl.TZ1.3b(ii):
Suggest a better method.
-
19M.3.sl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.sl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.sl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19N.3.sl.TZ0.5a:
Discuss why different methods of reduction are needed to extract metals.
-
19N.3.sl.TZ0.5b(ii):
Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
-
20N.3.sl.TZ0.4b(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020.
Write the half-equations for the reactions occurring in this electrolysis.
-
20N.3.sl.TZ0.4b(iii):
Calculate the theoretical mass of magnesium obtained if a current of is used for hours. Use charge and section 2 of the data booklet
-
20N.3.sl.TZ0.4b(iv):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
-
20N.3.hl.TZ0.4c(iii):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
-
20N.3.hl.TZ0.4c(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020
Calculate the theoretical mass of magnesium obtained if a current of 3.00 A is used for hours. Use charge :(Q) = current (I) × time (t) and section 2 of the data booklet.
A.3 Catalysts
- 16N.3.sl.TZ0.5a: Explain, with reference to their structure, the great selectivity of zeolites as catalysts.
-
16N.3.sl.TZ0.5b:
Nanocatalysts play an essential role in the manufacture of industrial chemicals.
(i) Describe the high pressure carbon monoxide (HIPCO) method for the production of carbon nanotubes.
(ii) Outline one benefit of using nanocatalysts compared to traditional catalysts in industry.
-
17M.3.sl.TZ1.9a:
Catalysts reduce the activation energy. Outline how homogeneous catalysts are involved in the reaction mechanism.
-
17M.3.sl.TZ1.9b:
Suggest why it is important to know how catalysts function.
-
17M.3.sl.TZ2.5a:
In a catalytic converter, carbon monoxide is converted to carbon dioxide. Outline the process for this conversion referring to the metal used.
-
17N.3.sl.TZ0.5:
Catalysts can take many forms and are used in many industrial processes.
Suggest two reasons why it might be worth using a more expensive catalyst to increase the rate of a reaction.
-
18M.3.sl.TZ1.4c.i:
The production of HDPE involves the use of homogeneous catalysts. Outline how homogeneous catalysts reduce the activation energy of reactions.
-
18M.3.sl.TZ2.3c.iii:
Vanadium(V) oxide is used as the catalyst in the conversion of sulfur dioxide to sulfur trioxide.
SO2(g) + V2O5(s) → SO3(g) + 2VO2(s)
O2(g) + 2VO2(s) → V2O5(s)
Outline how vanadium(V) oxide acts as a catalyst.
- 18N.3.sl.TZ0.2c.i: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
- 18N.3.sl.TZ0.2c.ii: Identify another advantage of using a zeolite instead of concentrated sulfuric acid.
- 18N.3.hl.TZ0.2c: A zeolite is an alternative catalyst for this reaction. Explain how zeolites act as selective...
-
19M.3.hl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.hl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19M.3.sl.TZ1.4a:
Outline two differences between heterogeneous and homogeneous catalysts.
-
19M.3.sl.TZ2.4c:
Explain the action of metals as heterogeneous catalysts.
-
19N.3.sl.TZ0.3a:
Describe how a heterogeneous catalyst provides an alternative pathway for a reaction.
-
19N.3.sl.TZ0.3c:
Nanotubes are used to support the active material in nanocatalysts.
Explain why oxygen cannot be used for the chemical vapour deposition (CVD) preparation of carbon nanotubes.
- 19N.3.sl.TZ0.3b: Distinguish between heterogeneous and homogeneous catalysts, giving one difference.
- 20N.3.sl.TZ0.4c: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
- 20N.3.hl.TZ0.4d: Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their...
A.4 Liquid crystals
- 16N.3.sl.TZ0.7a: Outline how a lyotropic liquid crystal differs from a thermotropic liquid crystal.
- 16N.3.sl.TZ0.7b: Explain the effect of increasing the temperature of a nematic liquid crystal on its directional...
-
17M.3.sl.TZ2.6a:
Two important properties of a liquid crystal molecule are being a polar molecule and having a long alkyl chain. Explain why these are essential components of a liquid crystal molecule.
-
17N.3.sl.TZ0.7a:
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
-
18M.3.hl.TZ2.6b:
MWCNT are very small in size and can greatly increase switching speeds in a liquid crystal allowing the liquid crystal to change orientation quickly.
Discuss two other properties a substance should have to be suitable for use in liquid crystal displays.
-
18M.3.sl.TZ1.3b.iv:
State the property of carbon nanotubes that enables them to form a nematic liquid crystal phase.
-
18M.3.sl.TZ1.4a:
Both of these are thermoplastic polymers. Outline what this term means.
-
18M.3.sl.TZ2.5b:
Discuss three properties a substance should have to be suitable for use in liquid crystal displays.
- 18N.3.sl.TZ0.4a: Outline two observations that he could have made.
- 18N.3.sl.TZ0.4b: The structure of biphenyl nitrile is shown. Describe, giving a reason, a feature of the...
-
19M.3.hl.TZ1.5a:
Label with an asterisk, *, the chiral carbon atom.
-
19M.3.hl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.hl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase.
Shape of molecules:
Distribution:
-
19M.3.sl.TZ1.5a:
State the name of the functional group which allows the molecule to be responsive to applied electric fields.
-
19M.3.sl.TZ1.5b:
Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
-
19M.3.sl.TZ2.3:
Describe the characteristics of the nematic liquid crystal phase and the effect that an electric field has on it.
Shape of molecules:Distribution:
Effect of electric field:
- 19N.3.sl.TZ0.6a: Describe the arrangement of soap molecules in the nematic liquid crystal phase.
- 19N.3.sl.TZ0.6b: State how liquid crystals are affected by an electric field.
- 20N.3.sl.TZ0.4d: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
- 20N.3.hl.TZ0.4e: Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how...
A.5 Polymers
-
16N.3.sl.TZ0.6a:
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
-
16N.3.sl.TZ0.6b:
Deduce the percentage atom economy for polymerization of 2-methylpropene.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
-
17M.3.sl.TZ1.10a:
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
-
17M.3.sl.TZ1.10b.i:
Describe the difference in their structures.
-
17M.3.sl.TZ1.10b.ii:
Explain why the difference in their structures affects their melting points.
-
17M.3.sl.TZ2.3c.i:
Explain how the structure of plasticizers enables them to soften PVC.
-
17M.3.sl.TZ2.3c.ii:
Suggest a reason why nanoparticles can better anchor plasticizers in the polymer.
-
17M.3.hl.TZ2.3c:
Estimate the atom economy of this first step.
-
17N.3.sl.TZ0.7b.i:
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
-
18M.3.hl.TZ1.4c.ii:
Deduce, giving a reason, whether the atom economy of a condensation polymerization, such as this, would be greater or less than an addition polymerization, such as the formation of HDPE.
-
18M.3.sl.TZ1.4a:
Both of these are thermoplastic polymers. Outline what this term means.
-
18M.3.sl.TZ1.4b.i:
Compare and contrast the structures of HDPE and LDPE.
-
18M.3.sl.TZ1.4b.ii:
State one way in which a physical property of HDPE, other than density, differs from that of LDPE as a result of this structural difference.
-
18M.3.sl.TZ2.4a:
Sketch four repeating units of the polymer to show atactic and isotactic polypropene.
-
18N.3.sl.TZ0.2b.i:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
-
18N.3.hl.TZ0.2b:
Structures of poly(methyl acrylate), PMA, and Bakelite® are shown.
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
-
19M.3.hl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.hl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.hl.TZ2.5c:
Explain how plasticizers affect the properties of plastics.
-
19M.3.sl.TZ1.4b:
Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
-
19M.3.sl.TZ2.5a:
Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
-
19M.3.sl.TZ2.5d:
Explain how plasticizers affect the properties of plastics.
- 19N.3.hl.TZ0.5b: State and explain why plasticizers are added to polymers.
-
19N.3.sl.TZ0.4a:
Draw a section of an isotactic polypropene polymer chain containing four repeating units.
- 19N.3.sl.TZ0.4b: Predict, with a reason, whether isotactic or atactic polypropene has the higher melting point.
- 19N.3.sl.TZ0.4c: Polypropene is a thermoplastic. Outline what is meant by thermoplastic.
- 19N.3.sl.TZ0.4d: Discuss why the recycling of plastics is an energy intensive process.
- 20N.3.sl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.sl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
- 20N.3.sl.TZ0.3c: Explain why phthalates are replaced by other plasticizers in the production of plastics.
- 20N.3.hl.TZ0.3b(i): Thermoplastic composites are increasingly replacing thermosets. Suggest one advantage of...
- 20N.3.hl.TZ0.3b(ii): Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the...
- 20N.3.hl.TZ0.3b(iii): Explain why phthalates are replaced by other plasticizers in the production of plastics.
A.6 Nanotechnology
-
16N.3.sl.TZ0.5b:
Nanocatalysts play an essential role in the manufacture of industrial chemicals.
(i) Describe the high pressure carbon monoxide (HIPCO) method for the production of carbon nanotubes.
(ii) Outline one benefit of using nanocatalysts compared to traditional catalysts in industry.
-
17M.3.sl.TZ1.8a:
State the major advantage that nanoparticles have in these applications.
-
17M.3.sl.TZ1.8b:
Suggest why nanoparticles need to be handled with care.
-
17N.3.sl.TZ0.6a:
State equations for the formation of iron nanoparticles and carbon atoms from Fe(CO)5 in the HIPCO process.
- 17N.3.sl.TZ0.6b: Outline why the iron nanoparticle catalysts produced by the HIPCO process are more efficient than...
- 17N.3.sl.TZ0.6c: Discuss one possible risk associated with the use of nanotechnology.
-
18M.3.sl.TZ1.3b.ii:
State one physical property of HDPE that will be affected by the incorporation of carbon nanotubes.
-
18M.3.sl.TZ1.3b.iii:
Describe how carbon nanotubes are produced by chemical vapour deposition (CVD).
-
18M.3.sl.TZ2.5a:
State the source of carbon for MWCNT produced by arc discharge and by CVD.
-
18N.3.sl.TZ0.4c:
Arc discharge, consisting of two inert metal electrodes in a liquid solvent, is one method of producing carbon nanotubes (CNTs).
Predict, giving a reason, the electrode at which the solvent cyclohexane, C6H12, will decompose to form CNTs.
-
19M.3.hl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.hl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.hl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19M.3.sl.TZ1.6a:
Describe the structure and bonding of a carbon nanotube.
Structure:
Bonding:
-
19M.3.sl.TZ1.6b:
Suggest one application for carbon nanotubes.
-
19M.3.sl.TZ2.4e:
Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
-
19N.3.sl.TZ0.3c:
Nanotubes are used to support the active material in nanocatalysts.
Explain why oxygen cannot be used for the chemical vapour deposition (CVD) preparation of carbon nanotubes.
- 20N.3.sl.TZ0.4a: Explain these properties of carbon nanotubes.
- 20N.3.hl.TZ0.4a: Explain these properties of carbon nanotubes.
A.7 Environmental impact—plastics
-
17N.3.sl.TZ0.7b.ii:
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
-
17N.3.sl.TZ0.7c:
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
-
18M.3.sl.TZ1.4d:
Suggest two of the major obstacles, other than collection and economic factors, which have to be overcome in plastic recycling.
-
18M.3.sl.TZ2.4b.i:
State the chemical reason why plastics do not degrade easily.
-
18M.3.sl.TZ2.4b.ii:
Compare two ways in which recycling differs from reusing plastics.
-
18N.3.sl.TZ0.2b.ii:
In an incomplete combustion of the polyvinyl chloride, PVC, it was found that hydrogen chloride, carbon monoxide, carbon dioxide, and water vapour were released.
Formulate an equation for this reaction using the formula of the PVC repeating unit.
-
18N.3.sl.TZ0.2d:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
-
19M.3.hl.TZ1.4c:
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.hl.TZ2.5d:
Suggest why the addition of plasticizers is controversial.
-
19M.3.sl.TZ1.4c(i):
Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
-
19M.3.sl.TZ1.4c(ii):
The trace quantities of dioxins from tyre fires are rarely inhaled and instead settle on the ground.
Describe why this is a health concern.
-
19M.3.sl.TZ2.5c:
Identify a hazardous product of the incineration of polychloroethene.
-
19M.3.sl.TZ2.5e:
Suggest why the addition of plasticizers is controversial.
- 19N.3.hl.TZ0.5c: Discuss why the recycling of plastics is an energy intensive process.
A.8 Superconducting metals and X-ray crystallography (HL only)
-
16N.3.hl.TZ0.8a:
(i) The diagram below shows the diffraction of two X-ray beams, y and z of wavelength λ, shining on a chromium crystal whose planes are a distance d nm apart.
Deduce the extra distance travelled by the second beam, z, compared to the first one, y.
(ii) State the Bragg’s condition for the observed diffraction to be at its strongest (constructive interference).
-
16N.3.hl.TZ0.8b:
(i) The mass of one unit cell of chromium metal is 17.28 × 10−23 g. Calculate the number of unit cells in one mole of chromium. Ar(Cr) = 52.00.
(ii) Deduce the number of atoms of chromium per unit cell.
- 16N.3.hl.TZ0.9a: Describe the Meissner effect.
- 16N.3.hl.TZ0.9b: Outline one difference between type 1 and type 2 superconductors.
-
17M.3.hl.TZ1.8a:
Lanthanum has a hexagonal close packed (hcp) crystal structure. State the coordination number of each lanthanum atom.
-
17M.3.hl.TZ1.8b:
Lanthanum becomes superconducting below 5 K. Explain, in terms of Bardeen–Cooper–Schrieffer (BCS) theory, how superconductivity occurs.
-
17M.3.hl.TZ1.8c:
Outline why superconductivity only occurs at low temperatures.
-
17M.3.hl.TZ2.5c.ii:
Rhodium is a type 1 superconductor.
Sketch graphs of resistance against temperature for a conductor and superconductor.

-
17M.3.hl.TZ2.5c.iii:
Contrast type 1 and type 2 superconductors by referring to three differences between them.
-
17N.3.hl.TZ0.6b:
Explain why Type 2 superconductors are generally more useful than Type 1.
-
17N.3.hl.TZ0.8a:
Calculate the total number of cobalt atoms within its unit cell.
-
17N.3.hl.TZ0.8b.i:
The atomic radius, r, of cobalt is 1.18 × 10–8 cm. Determine the edge length, in cm, of the unit cell, a, using the second diagram.
-
17N.3.hl.TZ0.8b.ii:
Determine a value for the density of cobalt, in g cm–3, using data from sections 2 and 6 of the data booklet and your answers from (a) and (b) (i).
If you did not obtain an answer to (b) (i), use 3.00 × 10–8 cm but this is not the correct answer.
-
18M.3.hl.TZ1.5b:
The diagram illustrates the crystal structure of aluminium metal with the unit cell indicated. Outline the significance of the unit cell.

-
18M.3.hl.TZ1.5c:
When X-rays of wavelength 0.154 nm are directed at a crystal of aluminium, the first order diffraction pattern is observed at 18°. Determine the separation of layers of aluminium atoms in the crystal, in m, using section 1 of the data booklet.
-
18M.3.hl.TZ1.5d.i:
Deduce what the shape of the graph indicates about aluminium.
-
18M.3.hl.TZ1.5d.ii:
Outline why the resistance of aluminium increases above 1.2 K.
-
18M.3.hl.TZ2.4a.i:
Deduce the number of atoms per unit cell in vanadium.
-
18M.3.hl.TZ2.4a.ii:
Calculate the expected first order diffraction pattern angle, in degrees, if x-rays of wavelength 150 pm are directed at a crystal of vanadium. Assume the edge length of the crystal to be the same as separation of layers of vanadium atoms found by x-ray diffraction. Use section 1 of the data booklet.
-
18M.3.hl.TZ2.4a.iii:
Calculate the average mass, in g, of a vanadium atom by using sections 2 and 6 of the data booklet.
-
18M.3.hl.TZ2.4a.iv:
Determine the volume, in cm3, of a vanadium unit cell.
-
18M.3.hl.TZ2.4a.v:
Determine the density, in g cm−3, of vanadium by using your answers to (a)(i), (a)(iii) and (a)(iv).
- 18N.3.hl.TZ0.5a.i: State the name of the crystal structure of gold.
-
18N.3.hl.TZ0.5a.ii:
Calculate the number of atoms per unit cell of gold, showing your working.
-
18N.3.hl.TZ0.5b:
The edge length of the gold unit cell is 4.08 × 10‒8 cm.
Determine the density of gold in g cm‒3, using sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ1.3d(i):
Lithium has shown some superconductive properties when doped into graphene or when under high pressure. Under high pressure, however, the Meissner effect is absent.
Describe the Meissner effect.
-
19M.3.hl.TZ1.3d(ii):
At very low temperatures, lithium atoms enhance the phonon binding of electrons in graphene suggesting the formation of Cooper pairs.
Explain how Cooper pairs are formed.
-
19M.3.hl.TZ1.3e:
Lithium forms a crystalline lattice with the unit cell structure shown below.
X-ray diffraction shows that the length of the edge of the unit cell is 3.51 × 10−8 cm.
Determine the density of lithium, in g cm−3, using sections 2 and 6 of the data booklet.
-
19M.3.hl.TZ2.6a:
State the number of atoms in the unit cell.
-
19M.3.hl.TZ2.6b:
Determine the density of calcium, in g cm−3, using section 2 of the data booklet.
Ar = 40.08; metallic radius (r) = 1.97 × 10−10 m
-
19M.3.hl.TZ2.7a:
State what is meant by a superconductor.
-
19M.3.hl.TZ2.18b:
Outline one approach to controlling industrial emissions of carbon dioxide.
- 19N.3.hl.TZ0.4a(i): Outline how resistance to electric currents occurs in metals.
- 19N.3.hl.TZ0.4a(ii): Suggest why the resistance of metals increases with temperature.
- 19N.3.hl.TZ0.4b: State two differences between Type I and Type II superconductors.
-
19N.3.hl.TZ0.7:
X-ray crystallography of a metal crystal produces a diffraction pattern of bright spots.
Using X-rays of wavelength 1.54 × 10−10 m, the first bright spots were produced at an angle θ of 22.3° from the centre.
Calculate the separation between planes of atoms in the lattice, in meters, using section 1 of the data booklet.
- 20N.3.hl.TZ0.4b(i): CNT can act as Type 2 superconductors. Outline why Type 2 superconductors are generally more...
-
20N.3.hl.TZ0.4b(ii):
Explain the role of electrons in superconducting materials in terms of the Bardeen–Cooper–Schrieffer (BCS) theory.
A.9 Condensation polymers (HL only)
-
16N.3.hl.TZ0.6d:
Fermentation of sugars from corn starch produces propane-1,3-diol, which can be polymerized with benzene-1,4-dicarboxylic acid to produce the PTT polymer (polytrimethylene terephthalate).
(i) Draw the molecular structure of each monomer.
(ii) Deduce the name of the linkage formed on polymerization between the two monomers and the name of the inorganic product.
-
17M.3.hl.TZ1.9a:
Deduce the repeating unit of the polymer and the other product of the reaction.
-
17M.3.hl.TZ1.9b:
State the class of polymer to which PETE belongs.
-
17M.3.hl.TZ2.3c.ii:
Suggest, giving one reason, whether this is an addition or condensation reaction.
- 17N.3.hl.TZ0.7b: Describe how the monomers of addition polymers and of condensation polymers differ.
-
17N.3.hl.TZ0.7c:
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
-
18M.3.hl.TZ1.4c.i:
Draw the structure of the monomer from which nylon-6 is produced by a condensation reaction.
-
18M.3.hl.TZ2.5c.i:
Distinguish between the manufacture of polyester and polyethene.
- 18N.3.hl.TZ0.2d.i: State the names of the two terminal functional groups in X.
- 18N.3.hl.TZ0.2d.ii: Deduce the repeating unit of the polymer of X.
-
18N.3.hl.TZ0.2d.iii:
Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
-
19M.3.hl.TZ1.4d:
Classify polybutadiene as either an addition or condensation polymer, giving a reason.
-
19M.3.hl.TZ1.4e:
State one factor considered when making green chemistry polymers.
-
19M.3.hl.TZ2.5e:
Outline, giving a reason, how addition and condensation polymerization compare with regard to green chemistry.
-
19M.3.hl.TZ2.5f:
Draw the full structural formula of the organic functional group formed during the polymerization of the two reactants below.
-
19N.3.hl.TZ0.5a:
Draw the structure of the monomers of Kevlar® if the by-product of the condensation polymerization is hydrogen chloride.
- 20N.3.hl.TZ0.3c: Classify PVC and polyethene terephthalate, PET, as addition or condensation polymers and deduce...
A.10 Environmental impact—heavy metals (HL only)
- 16N.3.hl.TZ0.10a: Compare and contrast the Fenton and Haber–Weiss reaction mechanisms.
-
16N.3.hl.TZ0.10b:
Adsorption and chelation are two methods of removing heavy metal ion pollution from the environment.
(i) Describe the process of adsorption.
(ii) Deduce the structure of the complex ion formed by the reaction of three H2N−CH2−CH2−NH2 chelating molecules with a mercury(II) ion.
-
17M.3.hl.TZ1.10b:
Hydrogen sulfide could be used to remove antimony(III) ions from a solution.
Determine the concentration of antimony(III) ions that would be required to precipitate antimony(III) sulfide in a solution saturated with hydrogen sulfide.
[S2−] in water saturated with hydrogen sulfide = 1.0 × 10−14 mol dm−3
Ksp (Sb2S3) = 1.6 × 10−93
-
17M.3.hl.TZ1.10c:
Identify a ligand that could be used to chelate antimony(III) ions in solution.
-
17M.3.hl.TZ2.4a:
Identify the other product formed.
-
17M.3.hl.TZ2.4b:
Explain why EDTA, a chelating agent, is more effective in removing heavy metal ions from solution than monodentate ligands.
-
17M.3.hl.TZ2.5b.iii:
Nickel(II) ions are least soluble at pH 10.5. Calculate the molar solubility of nickel(II) hydroxide at this pH. KspNi(OH)2 = 5.48 × 10–16.
- 17N.3.hl.TZ0.9a: State the name of one method, other than precipitation, of removing heavy metal ions from...
-
17N.3.hl.TZ0.9b:
The solubility product, Ksp , of cadmium sulfide, CdS, is 8.0 × 10–27. Determine the concentration of cadmium ions in 1.0 dm3 of a saturated solution of cadmium sulfide to which 0.10 mol of solid sodium sulfide has been added, stating any assumption you make.
-
18M.3.hl.TZ1.5e:
The concentration of aluminium in drinking water can be reduced by precipitating aluminium hydroxide. Calculate the maximum concentration of aluminium ions in water of pH 7 at 298 K. Solubility product of aluminium hydroxide = 3.3 × 10−34 at 298 K.
-
18M.3.hl.TZ2.4b.i:
Vanadium and other transition metals can interfere with cell metabolism.
State and explain one process, other than by creating free radicals, by which transition metals interfere with cell metabolism.
-
18M.3.hl.TZ2.4b.ii:
Vanadium(IV) ions can create free radicals by a Fenton reaction.
Deduce the equation for the reaction of V4+ with hydrogen peroxide.
-
18N.3.hl.TZ0.3b.ii:
Lead ions are toxic and can be precipitated using hydroxide ions.
Pb2+ (aq) + 2OH‒ (aq) Pb(OH)2 (s)
Sufficient sodium hydroxide solid is added to the antacid sample to produce a 1.0 × 10‒2 mol dm‒3 hydroxide ion solution at 298 K.
Deduce if a precipitate will be formed, using section 32 of the data booklet.
If you did not calculate the concentration of lead ions in (b)(i), use the value of 2.4 × 10−4 mol dm‒3, but this is not the correct value.
- 18N.3.hl.TZ0.3d.i: State one feature of a chelating agent.
-
18N.3.hl.TZ0.3d.ii:
An aqueous lead(II) ion reacts with three ethane-1,2-diamine molecules to form an octahedral chelate ion.
Outline why the chelate ion is more stable than the reactants.
-
19M.3.hl.TZ1.7a:
Explain how entropy affects this equilibrium.
-
19M.3.hl.TZ1.7b:
State the number of coordinate covalent bonds EDTA forms with Ni2+.
-
19M.3.hl.TZ2.8a:
Outline why heavy metals are toxic.
-
19M.3.hl.TZ2.8b:
Determine the maximum concentration of lead(II) ions at 298 K in a solution in which the concentration of carbonate ions is maintained at 1.10 × 10−4 mol dm−3. Use section 32 of the data booklet.
-
19M.3.hl.TZ2.8c:
State a method, other than precipitation, of removing heavy metal ions from solution.
-
19N.3.hl.TZ0.8:
1.40 × 10−3 g of NaOH (s) are dissolved in 250.0 cm3 of 1.00 × 10−11 mol dm−3 Pb(OH)2 (aq) solution.
Determine the change in lead ion concentration in the solution, using section 32 of the data booklet.
-
20N.3.hl.TZ0.5a:
Precipitation is one method used to treat waste water.
Phosphates, , in waste water can be removed by precipitation with magnesium ions. of magnesium phosphate is .
Calculate the maximum solubility of phosphate ions in a solution containing magnesium ions.
-
20N.3.hl.TZ0.5b:
Precipitation is one method used to treat waste water.
Zinc, cadmium, nickel, and lead are metal ions which can be removed by precipitation. Explain why waste water is adjusted to a pH of 9−10 to remove these ions by referring to section 32 of the data booklet.
