| Date | November 2018 | Marks available | 1 | Reference code | 18N.3.hl.TZ0.15 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | State | Question number | 15 | Adapted from | N/A |
Question
Chemical energy from redox reactions can be used as a source of electrical energy.
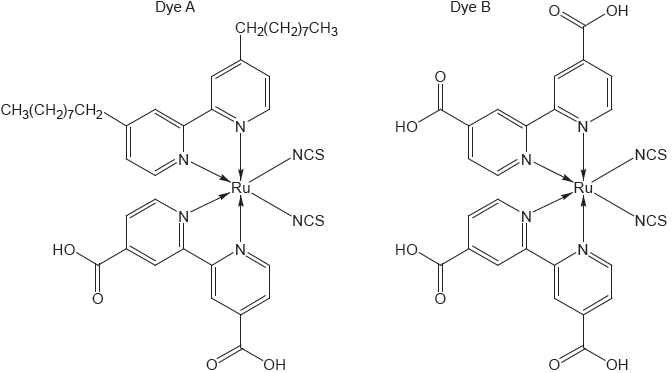
The chemical structure of a photosensitive dye found in blueberries and a schematic diagram of a solar cell are shown.
Outline how a rechargeable battery differs from a primary cell.
Formulate half-equations for the reactions at the anode (negative electrode) and cathode (positive electrode) during discharge of a lithium-ion battery.
A voltaic cell consists of a nickel electrode in 1.0 mol dm−3 Ni2+ (aq) solution and a cadmium electrode in a Cd2+ (aq) solution of unknown concentration.
Cd (s) + Ni2+ (aq) → Cd2+ (aq) + Ni (s) EΘcell = 0.14 V
Determine the concentration of the Cd2+ (aq) solution if the cell voltage, E, is 0.19 V at 298 K. Use section 1 of the data booklet.
Identify the structural feature of the dye that allows the conversion of solar energy into electrical energy.
Outline the effect of sunlight on the dye in the solar cell.
State the purpose of TiO2.
Deduce the reduction half-equation at the cathode.
Markscheme
«redox» reaction in rechargeable battery is reversible «but not in a primary cell»
OR
secondary cells need to be charged before use
OR
secondary cells have greater rate of self-discharge ✔
Accept “primary cells cannot be recharged/reused”, “primary cells can be used only once” OR “lithium batteries may explode”.
Anode (negative electrode):
Li (graphite) → Li+ (electrolyte) + e–
OR
LiC6 (s) → 6C (s) + Li+ (electrolyte) + e– ✔
Cathode (positive electrode):
Li+ (electrolyte) + e– + MnO2 (s) → LiMnO2 (s)
OR
Li+ (electrolyte) + e– + NiO2 (s) → LiNiO2 (s)
OR
Li+ (electrolyte) + e– + CoO2 (s) → LiCoO2 (s)
OR
2Li+ (electrolyte) + 2e– + 2CoO2 (s) → Co2O3 (s) + Li2O (s) ✔
Accept “polymer” for “electrolyte”.
Award [1 max] if electrodes are reversed.
Do not accept “CO” for “Co”.
«E = EΘ − lnQ»
OR
0.05 = –0.01283 ln [Cd2+]
OR
ln[Cd2+] = – 3.897 ✔
[Cd2+] = 0.020 «mol dm–3» ✔
Award [2] for correct final answer.
«extensive» conjugation
OR
«extensive» alternate single and double bonds ✔
Accept “delocalization”.
electrons excited/released «from dye» ✔
Accept “photooxidation/oxidizes dye”.
transfers e– to external circuit ✔
Accept “provides large surface area”.
I3– (aq) + 2e– → 3I– (aq) ✔
Accept “3I2 (aq) + 2e– → 2I3– (aq)”.