| Date | May 2019 | Marks available | 2 | Reference code | 19M.3.hl.TZ2.13 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Outline | Question number | 13 | Adapted from | N/A |
Question
Hemoglobin contains heme groups with the porphyrin ring bound to an iron(II) ion.
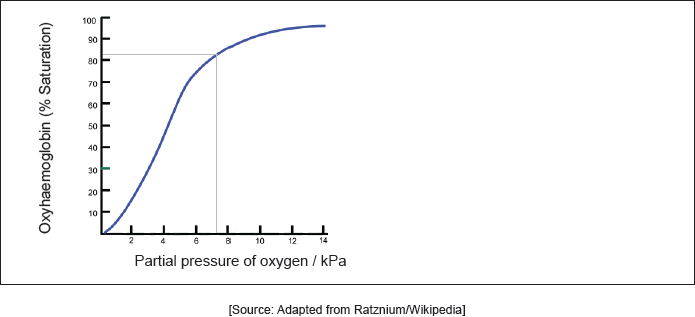
A hemoglobin’s oxygen dissociation curve is shown.
Outline why the complex formed between Fe2+ and oxygen is red. Refer to the diagram above and section 17 of the data booklet.
Explain the shape of the curve.
Sketch another line to show the effect of an increase in body temperature on the oxygen saturation of hemoglobin.
Markscheme
extensive conjugated system
OR
extensive delocalized bonding system
OR
extended system of alternating double and single bonds [✔]
absorbs green
OR
complementary to red light [✔]
sigmoid/S-shaped
OR
as oxygen binds to one active site, shape of other active sites change [✔]
affinity of other sites for oxygen increases/ability to bind oxygen is increased by initial binding of oxygen
OR
cooperative binding [✔]
Note: Accept description of sigmoid/S-shaped curve if not stated for M1.
Examiners report
This part was fairly well answered with the majority of the candidates managed one mark. The second mark was missed because the candidates did not write extensive conjugated system or extensive delocalised bonding system.
Many candidates managed one mark by describing the graph and stating that the affinity of other sites for oxygen increases/cooperative bonding. Several candidates missed that it was a sigmoid/S-shaped curve.
Many candidates were able to sketch another line to show the effect of an increase in body temperature on the oxygen saturation of haemoglobin.