DP Chemistry Questionbank

C: Energy
Description
[N/A]Directly related questions
-
16N.3.hl.TZ0.21a:
The Geobacter species of bacteria can be used in microbial fuel cells to oxidise aqueous ethanoate ions,
CH3COO−(aq), to carbon dioxide gas.State the half-equations for the reactions at both electrodes.
-
16N.3.hl.TZ0.21c:
Dye-sensitized solar cells (DSSC) convert solar energy into electrical energy.
(i) Describe how a DSSC converts sunlight into electrical energy.
(ii) Explain the role of the electrolyte solution containing iodide ions, I−, and triiodide ions, I3−, in the DSSC.
-
16N.3.sl.TZ0.11a:
(i) Calculate the specific energy of the lithium ion battery, in MJ kg−1, when 80.0 kg of fuel in the battery releases 1.58 × 107 J. Use section 1 of the data booklet.
(ii) The specific energy of gasoline is 46.0 MJ kg−1. Suggest why gasoline may be considered a better energy source than the lithium ion battery based on your answer to part (a) (i).
-
16N.3.sl.TZ0.13a:
Explain the effect of the increasing concentration of atmospheric carbon dioxide on the acidity of oceans.
- 16N.3.sl.TZ0.14a: State the equation for the complete transesterification of the triglyceride given below with...
-
16N.3.sl.TZ0.13b:
(i) Describe the changes that occur at the molecular level when atmospheric carbon dioxide gas absorbs infrared radiation emitted from the Earth’s surface.
(ii) Other than changes to the acidity of oceans, suggest why the production of carbon dioxide is of greater concern than the production of water vapour.
-
16N.3.sl.TZ0.15b:
Radioactive phosphorus, 33P, has a half-life of 25.3 days.
(i) Calculate 33P decay constant λ and state its unit. Use section 1 of the data booklet.
(ii) Determine the fraction of the 33P sample remaining after 101.2 days.
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
- 16N.3.sl.TZ0.12a: Discuss how the octane number changes with the molecular structure of the alkanes.
-
16N.3.sl.TZ0.11b:
(i) The energy density of gasoline is 34.3 MJ dm−3. Calculate the volume of gasoline, in dm3, that is equivalent to the energy in 80.0 kg of fuel in the lithium ion battery. Use section 1 of the data booklet.
(ii) The efficiency of energy transfer by this lithium ion battery is four times greater than that of gasoline. Determine the distance, in km, the car can travel on the lithium ion battery power alone if the gasoline-powered car uses 1.00 dm3 gasoline to travel 32.0 km.
- 16N.3.sl.TZ0.12b: Catalytic reforming and cracking reactions are used to produce more efficient fuels. Deduce the...
-
16N.3.sl.TZ0.14b:
Outline why the fuel produced by the reaction in (a) is more suitable for use in diesel engines than vegetable oils.
-
16N.3.sl.TZ0.15a:
(i) Explain why fusion, combining two smaller nuclei into a larger nucleus, releases vast amounts of energy. Use section 36 of the data booklet.
(ii) Outline one advantage of fusion as a source of energy.
- 20N.3.sl.TZ0.9e: Contrast the molecular structures of biodiesel and the vegetable oil from which it is formed.
-
20N.3.sl.TZ0.9a:
Calculate the energy released, in , from the complete combustion of of ethanol.
-
20N.3.sl.TZ0.9d:
A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
-
20N.3.sl.TZ0.9c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
-
20N.3.sl.TZ0.9f(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
- 20N.3.sl.TZ0.10e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
- 20N.3.sl.TZ0.9b: State a class of organic compounds found in gasoline.
-
20N.3.sl.TZ0.10b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
- 20N.3.sl.TZ0.10c: Outline a health risk produced by exposure to radioactive decay.
-
20N.3.sl.TZ0.10d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
-
20N.3.sl.TZ0.9f(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
- 20N.3.hl.TZ0.14b: Doping of silicon increases the conductivity in semiconductors. Explain how doping improves the...
-
20N.3.hl.TZ0.11a:
Calculate the energy released, in , from the complete combustion of of ethanol.
- 20N.3.hl.TZ0.11d: A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture...
- 20N.3.hl.TZ0.12e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
-
20N.3.hl.TZ0.11e(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
- 20N.3.hl.TZ0.11b: State a class of organic compounds found in gasoline.
-
20N.3.hl.TZ0.12f:
Determine the nuclear binding energy, in , of using sections 2 and 4 of the data booklet.
The mass of the nucleus is .
- 20N.3.hl.TZ0.12c: Outline a health risk produced by exposure to radioactive decay.
-
20N.3.hl.TZ0.11e(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
-
20N.3.hl.TZ0.12b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
-
20N.3.hl.TZ0.12d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
-
20N.3.hl.TZ0.11c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
-
20N.3.hl.TZ0.11e(iv):
Determine the relative rate of effusion of methane () to carbon dioxide (), under the same conditions of temperature and pressure. Use section 1 of the data booklet.
-
20N.3.hl.TZ0.14a:
Doping of silicon increases the conductivity in semiconductors.
Describe the doping in p-type and n-type semiconductors.
-
17M.3.sl.TZ1.14a:
Outline how the spectra of light from stars can be used to detect the presence of carbon.
-
17M.3.sl.TZ1.14b.i:
Deduce the identity of X.
-
17M.3.sl.TZ1.14b.ii:
Outline why this reaction results in a release of energy.
-
17M.3.sl.TZ1.14c:
Nuclear fusion reactors are predicted to become an important source of electrical energy in the future. State two advantages of nuclear fusion over nuclear fission.
-
17M.3.sl.TZ1.15a:
State two reagents required to convert vegetable oil to biodiesel.
-
17M.3.sl.TZ1.15b:
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
-
17M.3.sl.TZ1.15c:
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
-
17M.3.sl.TZ1.15d:
Determine the specific energy, in kJg−1, and energy density, in kJcm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 gcm−3; Molar mass = 299 gmol−1;
Enthalpy of combustion = 12.0 MJmol−1.
-
17M.3.sl.TZ1.16a:
State how these gases are produced, giving the appropriate equation(s).
-
17M.3.sl.TZ1.16b:
Outline how the carbon monoxide is then converted to a hydrocarbon fuel.
-
17M.3.sl.TZ1.17a:
Suggest why it is only in recent years that specific predictions of the future effects of fossil fuel combustion have been made.
-
17M.3.sl.TZ1.17b:
Carbon dioxide has two different bond stretching modes illustrated below.

Predict, with an explanation, whether these stretching modes will absorb infrared radiation.
-
17M.3.sl.TZ1.17c:
Outline, giving the appropriate equation(s), how increasing levels of carbon dioxide will affect the pH of the oceans.
-
17M.3.sl.TZ1.17d:
Many combustion processes also release particulate matter into the atmosphere. Suggest, giving your reason, how this might affect the temperature of the Earth’s surface.
-
17M.3.hl.TZ1.18b.ii:
The mass of X is 8.005305 amu and that of is 4.002603 amu. Determine the energy produced, in J, when one atom of is formed in this reaction. Use section 2 of the data booklet.
-
17M.3.hl.TZ1.19a:
Identify two ways in which the structure of the dye shown resembles the chlorophyll molecule. Use section 35 of the data booklet.
-
17M.3.hl.TZ1.19b:
Both photosynthesis and the Grätzel cell use energy from sunlight to bring about reduction. Deduce an equation for the reduction reaction in the electrolyte of a Grätzel cell.
-
17M.3.hl.TZ1.22a:
Deduce half-equations for the reactions at the two electrodes and hence the equation for the overall reaction.
-
17M.3.hl.TZ1.22b.i:
Suggest a way in which they are similar.
-
17M.3.hl.TZ1.22b.ii:
Outline the difference between primary and rechargeable cells.
-
17M.3.hl.TZ1.22c:
Identify one factor that affects the voltage of a cell and a different factor that affects the current it can deliver.
-
17M.3.sl.TZ2.12b:
Coloured molecules absorb sunlight. Identify the bonding characteristics of such molecules.
-
17M.3.sl.TZ2.13a:
State one advantage and one disadvantage for each energy source in the table.
-
17M.3.sl.TZ2.13b.i:
Calculate the specific energy of hydrogen, stating its units. Refer to sections 1, 6 and 13 of the data booklet.
-
17M.3.sl.TZ2.13b.ii:
Hydrogen has a higher specific energy than petrol (gasoline) but is not used as a primary fuel source in cars. Discuss the disadvantages of using hydrogen.
-
17M.3.sl.TZ2.14a:
Identify which region, A or B, corresponds to each type of radiation by completing the table.
-
17M.3.sl.TZ2.14b.i:
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
-
17M.3.sl.TZ2.14b.ii:
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
-
17M.3.sl.TZ2.14c.i:
Suggest an equation for the production of syngas from coal.
-
17M.3.sl.TZ2.14c.ii:
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
-
17M.3.sl.TZ2.14c.iii:
Suggest a reason why syngas may be considered a viable alternative to crude oil.
-
17M.3.sl.TZ2.12a.i:
One fusion reaction occurring in the sun is the fusion of deuterium, , with tritium, , to form helium, . State a nuclear equation for this reaction.
-
17M.3.sl.TZ2.12a.ii:
Explain why this fusion reaction releases energy by using section 36 of the data booklet.
-
17M.3.hl.TZ2.16a.iii:
Calculate the energy released, in MeV, in this reaction, using section 36 of the data booklet.
-
17M.3.hl.TZ2.17c.i:
Deduce the half-cell equations occurring at each electrode during discharge.
-
17M.3.hl.TZ2.17c.ii:
Outline the function of the proton-exchange membrane (PEM) in the fuel cell.
-
17M.3.hl.TZ2.17c.iii:
Explain how the flow of ions allows for the operation of the fuel cell.
-
17M.3.hl.TZ2.18a.ii:
The structures of 11-cis-retinal and β-carotene are given in section 35 of the data booklet. Suggest a possible wavelength of light absorbed by each molecule using section 3 of the data booklet.
-
17M.3.hl.TZ2.19a:
Contrast how absorption of photons and charge separation occur in each device.
-
17M.3.hl.TZ2.19b:
Suggest one advantage a DSSC has over a silicon based photovoltaic cell.
-
17N.3.hl.TZ0.18c.i:
Calculate the loss in mass, in kg, and the energy released, in J, when 0.00100 mol of 228Ac decays, each atom losing an electron. Use section 2 of the data booklet and E = mc2.
228Ac → + 228Th
-
17N.3.hl.TZ0.18c.ii:
Determine the energy released, in J, by 0.00100 mol of 228Ac over the course of 18 hours.
- 17N.3.hl.TZ0.18d: Outline how nuclear ionising radiation can damage DNA and enzymes in living cells.
-
17N.3.hl.TZ0.20b:
Outline one advantage and one disadvantage of the methanol cell (DMFC) compared with a hydrogen-oxygen fuel cell.
-
17N.3.sl.TZ0.12b:
A typical wood has a specific energy of 17 × 103 kJ kg–1. Comment on the usefulness of octane and wood for powering a moving vehicle, using your answer to (a).
If you did not work out an answer for (a), use 45 × 103 kJ kg–1 but this is not the correct answer.
-
17N.3.hl.TZ0.19b:
The natural absorption of light by chlorophyll has been copied by those developing dye-sensitized solar cells (DSSCs). Outline how a DSSC works.
-
17N.3.hl.TZ0.20a:
Deduce the half-equations and the overall equation for the reactions taking place in a direct methanol fuel cell (DMFC) under acidic conditions.
-
17N.3.sl.TZ0.13a:
“Knocking” in an automobile (car) engine can be prevented by increasing the octane number of the fuel. Explain, including an equation with structural formulas, how heptane, C7H16, could be chemically converted to increase its octane number.
- 17N.3.sl.TZ0.13d: Outline how water and carbon dioxide absorb infrared radiation.
-
17N.3.sl.TZ0.13b:
Many like to refer to our “carbon footprint”. Outline one difficulty in quantifying such a concept.
-
17N.3.sl.TZ0.14b:
The amount of 228Ac in a sample decreases to one eighth of its original value in about 18 hours due to β-decay. Estimate the half-life of 228Ac.
- 17N.3.sl.TZ0.15a: State the structural feature of chlorophyll that enables it to absorb visible light.
-
17N.3.sl.TZ0.12a:
Calculate the specific energy of octane, C8H18, in kJ kg–1 using sections 1, 6 and 13 of the data booklet.
-
17N.3.sl.TZ0.13c:
Climate change or global warming is a consequence of increased levels of carbon dioxide in the atmosphere. Explain how the greenhouse effect warms the surface of the earth.
-
17N.3.sl.TZ0.14a.i:
Compare and contrast fission and fusion in terms of binding energy and the types of nuclei involved.
-
17N.3.sl.TZ0.14a.ii:
Suggest two advantages that fusion has over fission.
-
17N.3.sl.TZ0.12c:
State the name of one renewable source of energy other than wood.
- 17N.3.sl.TZ0.15b: Vegetable oils are too viscous for use as liquid fuels. Describe, using an equation, how a...
-
18M.3.hl.TZ1.15b:
Dye-sensitized solar cells, DSSCs, use a dye to absorb the sunlight. State two advantages that DSSCs have over traditional silicon based photovoltaic cells.
-
18M.3.hl.TZ1.13a:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
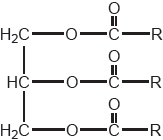
18M.3.hl.TZ1.13b:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
-
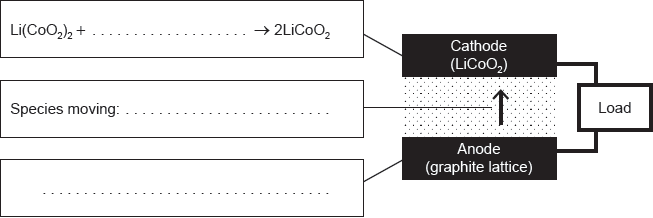
18M.3.hl.TZ1.14a.i:
Complete the half-equations on the diagram and identify the species moving between the electrodes.
-
18M.3.hl.TZ1.14a.ii:
State the factor that limits the maximum current that can be drawn from this cell and how electrodes are designed to maximize the current.
-
18M.3.hl.TZ1.14b.ii:
Explain how the proportion of 235U in natural uranium is increased.
-
18M.3.hl.TZ1.15a:
Early photovoltaic cells were based on silicon containing traces of other elements. State the type of semiconductor produced by doping silicon with indium, In, giving a reason that refers to its electronic structure.
-
18M.3.hl.TZ1.15c:
The structure of two dyes used in DSSCs are shown.
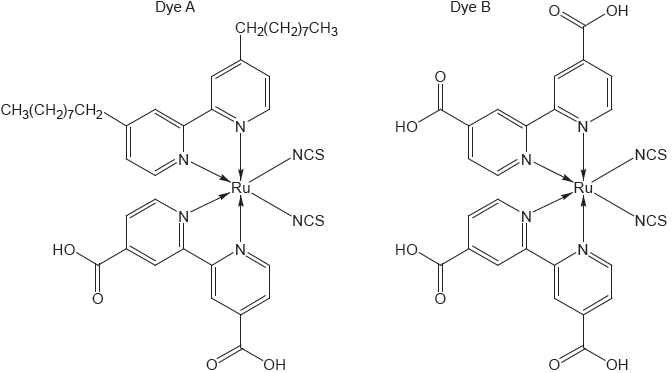
Predict, giving a reason, which dye will absorb light of longer wavelength.
-
18M.3.hl.TZ2.13c:
Fuel cells have a higher thermodynamic efficiency than octane. The following table gives some information on a direct methanol fuel cell.

Determine the thermodynamic efficiency of a methanol fuel cell operating at 0.576 V.
Use sections 1 and 2 of the data booklet.
-
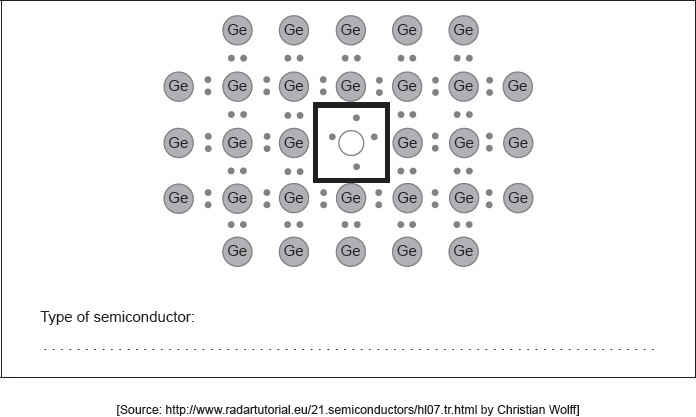
18M.3.hl.TZ2.18a:
Draw the Lewis (electron dot) structure for an appropriate doping element in the box in the centre identifying the type of semiconductor formed.
-
18M.3.hl.TZ2.18b.ii:
Outline why complex B absorbs light of longer wavelength than complex A.
-
18M.3.hl.TZ2.16c.i:
Calculate the relative rate of effusion of 235UF6(g) to 238UF6(g) using sections 1 and 6 of the data booklet.
-
18M.3.hl.TZ2.18b.i:
State the feature of the molecules responsible for the absorption of light.
-
18M.3.hl.TZ2.16c.ii:
Explain, based on molecular structure and bonding, why diffusion or centrifuging can be used for enrichment of UF6 but not UO2.
-
18M.3.sl.TZ1.9a:
Identify one naturally occurring greenhouse gas, other than carbon dioxide or water vapour, and its natural source.
-
18M.3.sl.TZ1.11a.ii:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
-
18M.3.sl.TZ1.11b:
Outline why biofuels are considered more environmentally friendly, even though they produce more carbon dioxide per kJ of energy than petroleum based fuels.
-
18M.3.sl.TZ1.10c.i:
Determine the specific energy and energy density of petrol (gasoline), using data from sections 1 and 13 of the data booklet. Assume petrol is pure octane, C8H18. Octane: molar mass = 114.26 g mol−1, density = 0.703 g cm−3.
-
18M.3.sl.TZ1.9c:
The concentrations of oxygen and nitrogen in the atmosphere are much greater than those of greenhouse gases. Outline why these gases do not absorb infrared radiation.
-
18M.3.sl.TZ1.10a:
Identify an element, other than carbon and hydrogen, found at significant concentrations in fossil fuels.
-
18M.3.sl.TZ1.10b:
Petroleum contains many hydrocarbons. Explain how these are separated by fractional distillation.
-
18M.3.sl.TZ1.10c.ii:
Outline why the energy available from an engine will be less than these theoretical values.
-
18M.3.sl.TZ1.11a.i:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
18M.3.sl.TZ1.12b:
Suggest one reason why there is opposition to the increased use of nuclear fission reactors.
-
18M.3.sl.TZ1.9b:
Formulate an equation that shows how aqueous carbon dioxide produces hydrogen ions, H+(aq).
-
18M.3.sl.TZ2.11a:
Explain the molecular mechanism by which carbon dioxide acts as a greenhouse gas.
-
18M.3.sl.TZ2.12b:
Power plants generating electricity by burning coal to boil water operate at approximately 35% efficiency.
State what this means and suggest why it is lower than the thermal efficiency.
-
18M.3.sl.TZ2.14a:
Deduce the equation for the transesterification reaction of pentyl octanoate, C7H15COOC5H11, with methanol.
-
18M.3.sl.TZ2.10c.i:
Outline how higher octane fuels help eliminate “knocking” in engines.
-
18M.3.sl.TZ2.10a:
Outline two reasons why oil is one of the world’s significant energy sources.
-
18M.3.sl.TZ2.10b.i:
Formulate an equation for the cracking of C16H34 into two products with eight carbon atoms each.
-
18M.3.sl.TZ2.10b.ii:
Identify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
-
18M.3.sl.TZ2.10c.ii:
The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
-
18M.3.sl.TZ2.11b:
Discuss the significance of two greenhouse gases, other than carbon dioxide, in causing global warming or climate change.
-
18M.3.sl.TZ2.12a:
Calculate the thermal efficiency of a steam turbine supplied with steam at 540°C and using a river as the choice of sink at 23 °C.
-
18M.3.sl.TZ2.13a:
Compare and contrast the process of nuclear fusion with nuclear fission.
-
18M.3.sl.TZ2.13b:
Dubnium-261 has a half-life of 27 seconds and rutherfordium-261 has a half-life of 81 seconds.
Estimate what fraction of the dubnium-261 isotope remains in the same amount of time that of rutherfordium-261 decays.
-
18M.3.sl.TZ2.14b:
Outline why the ester product of this reaction is a better diesel fuel than pentyl octanoate.
-
18M.3.sl.TZ1.12a.ii:
Explain how 235U fission results in a chain reaction, including the concept of critical mass.
-
18N.3.sl.TZ0.10a:
Formulate equation(s) for the conversion of coal and steam to methane.
-
18N.3.hl.TZ0.13a:
Formulate equation(s) for the conversion of coal and steam to methane.
-
18N.3.hl.TZ0.15d.iv:
Deduce the reduction half-equation at the cathode.
-
18N.3.sl.TZ0.9c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
-
18N.3.hl.TZ0.13c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
- 18N.3.sl.TZ0.11a: Suggest another advantage and one disadvantage of solar energy.
- 18N.3.hl.TZ0.15d.ii: Outline the effect of sunlight on the dye in the solar cell.
- 18N.3.sl.TZ0.9b.i: Outline why the term breeder is used for the reactors.
-
18N.3.sl.TZ0.11e:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
-
18N.3.sl.TZ0.10b.ii:
Comment on the specific energies of hydrogen and methane.
-
18N.3.sl.TZ0.10b.i:
Calculate the specific energy, in kJ g−1, of methane.
-
18N.3.hl.TZ0.14d:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
- 18N.3.sl.TZ0.11c.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
- 18N.3.sl.TZ0.11d: Contrast the importance of carbon dioxide and methane as greenhouse gases.
- 18N.3.hl.TZ0.12d.ii: Explain why free radicals are harmful to living cells.
-
18N.3.hl.TZ0.13b:
Comment on the specific energies of hydrogen and methane.
-
18N.3.hl.TZ0.15c:
A voltaic cell consists of a nickel electrode in 1.0 mol dm−3 Ni2+ (aq) solution and a cadmium electrode in a Cd2+ (aq) solution of unknown concentration.
Cd (s) + Ni2+ (aq) → Cd2+ (aq) + Ni (s) EΘcell = 0.14 V
Determine the concentration of the Cd2+ (aq) solution if the cell voltage, E, is 0.19 V at 298 K. Use section 1 of the data booklet.
- 18N.3.hl.TZ0.15d.i: Identify the structural feature of the dye that allows the conversion of solar energy into...
-
18N.3.hl.TZ0.12d.i:
Deduce a Lewis (electron dot) structure of the superoxide, O2–, free radical.
- 18N.3.sl.TZ0.11b: Light can be absorbed by chlorophyll and other pigments. Consider molecules A and B represented...
- 18N.3.hl.TZ0.14c: Contrast the importance of carbon dioxide and methane as greenhouse gases.
- 18N.3.sl.TZ0.11c.ii: Describe how vegetable oils can be converted to a more suitable fuel.
- 18N.3.sl.TZ0.9a: Explain fusion reactions with reference to binding energy.
-
18N.3.sl.TZ0.10c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
- 18N.3.hl.TZ0.12a: Explain fusion reactions with reference to binding energy.
-
18N.3.hl.TZ0.12c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
- 18N.3.hl.TZ0.14b.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
- 18N.3.hl.TZ0.15d.iii: State the purpose of TiO2.
- 18N.3.hl.TZ0.12b.i: Outline why the term breeder is used for the reactors.
-
18N.3.hl.TZ0.15b:
Formulate half-equations for the reactions at the anode (negative electrode) and cathode (positive electrode) during discharge of a lithium-ion battery.
- 18N.3.sl.TZ0.9b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
- 18N.3.hl.TZ0.12b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
- 18N.3.hl.TZ0.14a: Suggest another advantage and one disadvantage of solar energy.
- 18N.3.hl.TZ0.14b.ii: Describe how vegetable oils can be converted to a more suitable fuel.
- 18N.3.hl.TZ0.15a: Outline how a rechargeable battery differs from a primary cell.
-
19M.3.hl.TZ1.16a(ii):
Outline why the reaction releases energy.
-
19M.3.hl.TZ1.15c(i):
Methane can also be obtained by fractional distillation of crude oil.
[Source: Image used with kind permission of science-resources.co.uk]
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.hl.TZ1.16a(iii):
The masses of the particles involved in this fission reaction are shown below.
Mass of neutron = 1.00867 amu
Mass of U-235 nucleus = 234.99346 amu
Mass of Ba-144 nucleus = 143.89223 amu
Mass of Kr-89 nucleus = 88.89788 amuDetermine the energy released, in J, when one uranium-235 nucleus undergoes fission. Use this data and information from sections 1 and 2 of the data booklet.
-
19M.3.hl.TZ1.15d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.hl.TZ1.15b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.hl.TZ1.15b(ii):
Hydroelectric power plants produced 16% of the world’s energy in 2015, down from 21% in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.hl.TZ1.17b(i):
Ethanol can be used in a direct-ethanol fuel cell (DEFC) as illustrated by the flow chart.
Deduce the half-equations occurring at electrodes A and B.
Electrode A:
Electrode B:
-
19M.3.hl.TZ1.15d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.hl.TZ1.17c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.hl.TZ1.18a:
Some solar cells use photovoltaic semi-conductors. Compare, giving reasons, the electrical conductivity of metals and semi-conductors as temperature increases.
-
19M.3.hl.TZ1.16b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.hl.TZ1.17b(ii):
State the name and function of X in the diagram in (b)(i).
Name:
Function:
-
19M.3.hl.TZ1.15a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.hl.TZ1.15c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.hl.TZ1.16c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for its radioactivity to fall to 10% of its initial value, using section 1 of the data booklet.
-
19M.3.hl.TZ1.17b(iii):
Outline why aqueous ethanol, rather than pure ethanol, is used in a DEFC.
-
19M.3.hl.TZ2.16a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.hl.TZ1.17a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.hl.TZ1.18b:
Suggest one advantage of a dye-sensitized solar cell (DSSC) over a silicon based photovoltaic cell.
-
19M.3.hl.TZ1.16a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.hl.TZ2.19a:
Outline how a microbial fuel cell produces an electric current from glucose.
C6H12O6 (aq) + 6O2 (g) → 6CO2 (g) + 6H2O (l)
- 19M.3.hl.TZ2.19c: Outline one difference between a primary and a secondary cell.
-
19M.3.hl.TZ2.20a:
Sketch graphs to show the general effect of increasing temperature on the electrical conductivity of semiconductors and metals on the axes below.
-
19M.3.hl.TZ2.15a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.hl.TZ2.15c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
-
19M.3.hl.TZ2.16d:
Calculate the half-life of an isotope whose mass falls from 5.0 × 10−5 g to 4.0 × 10−5 g in 31.4 s, using section 1 of the data booklet.
-
19M.3.hl.TZ2.19b:
The cell potential for the spontaneous reaction when standard magnesium and silver half-cells are connected is +3.17 V.
Determine the cell potential at 298 K when:
[Mg2+] = 0.0500 mol dm−3
[Ag+] = 0.100 mol dm−3Use sections 1 and 2 of the data booklet.
-
19M.3.hl.TZ2.15b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19M.3.hl.TZ2.18b:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.hl.TZ2.16a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.hl.TZ2.14:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.hl.TZ2.16a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.hl.TZ2.20b:
Explain the function of dyes in a dye-sensitized solar cell (DSSC).
-
19M.3.hl.TZ2.16b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
-
19M.3.hl.TZ2.16c:
Outline how the energy of a fission reaction can be calculated.
-
19M.3.hl.TZ2.18a:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.hl.TZ2.17:
This question is about biofuel.
Evaluate the use of biodiesel in place of diesel from crude oil.
-
19M.3.sl.TZ1.11b(ii):
Hydroelectric power plants produced 16 % of the world’s energy in 2015, down from 21 % in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.sl.TZ1.11c(i):
Methane can also be obtained by fractional distillation of crude oil.
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.sl.TZ1.13c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.sl.TZ1.11a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.sl.TZ1.11d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.sl.TZ1.13a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.sl.TZ1.12c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for the mass of 89Kr to fall to 6.25 % of its initial value.
-
19M.3.sl.TZ1.11b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.sl.TZ1.11c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.sl.TZ1.12a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.sl.TZ1.11d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.sl.TZ1.12a(ii):
Outline why the reaction releases energy.
-
19M.3.sl.TZ1.13b:
Show that, for combustion of equal masses of fuel, ethanol (Mr = 46 g mol−1) has a lower carbon footprint than octane (Mr = 114 g mol−1).
-
19M.3.sl.TZ1.12b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.sl.TZ2.10c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
-
19M.3.sl.TZ2.10b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19M.3.sl.TZ2.12b:
Evaluate the use of biodiesel in place of diesel from crude oil.
Strength:
Limitation:
-
19M.3.sl.TZ2.13c:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.sl.TZ2.13a:
State one greenhouse gas, other than carbon dioxide.
-
19M.3.sl.TZ2.9:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.sl.TZ2.10a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.sl.TZ2.12a:
The structure of chlorophyll is given in section 35 of the data booklet.
State the feature of the chlorophyll molecule that enables it to absorb light in the visible spectrum.
-
19M.3.sl.TZ2.11a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.sl.TZ2.11c:
90Sr, a common product of fission, has a half-life of 28.8 years.
Determine the number of years for the activity of a sample of 90Sr to fall to one eighth () of its initial value.
-
19M.3.sl.TZ2.13b:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.sl.TZ2.11a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.sl.TZ2.11a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.sl.TZ2.11b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
- 19N.3.hl.TZ0.16b: Outline what is meant by the degradation of energy.
-
19N.3.hl.TZ0.18a(iii):
Calculate the heat energy released, in J, by the fusion reaction producing one atom of carbon-12. Use section 2 of the data booklet and E = mc2.
-
19N.3.sl.TZ0.13b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
- 19N.3.hl.TZ0.20d(i): Outline the functions of the dye, TiO2 and the electrolyte in the operation of the...
- 19N.3.sl.TZ0.12b(ii): The 1H NMR spectrum of one of the products has four signals. The integration trace shows a ratio...
-
19N.3.hl.TZ0.20b(ii):
Suggest how PEM fuel cells can be used to produce a larger voltage than that calculated in (b)(i).
-
19N.3.sl.TZ0.11a:
Discuss the data.
- 19N.3.sl.TZ0.12b(i): Reforming reactions are used to increase the octane number of a hydrocarbon fuel. Suggest the...
- 19N.3.sl.TZ0.13a(ii): Explain why fusion is an exothermic process.
-
19N.3.sl.TZ0.14a:
Write the equation for the complete combustion of ethanol.
-
19N.3.sl.TZ0.14b:
Outline the evidence that relates global warming to increasing concentrations of greenhouse gases in the atmosphere.
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
- 19N.3.hl.TZ0.20d(ii): Suggest an advantage of the DSSC over silicon-based photovoltaic cells.
-
19N.3.sl.TZ0.11b:
In a natural gas power station, 1.00 tonne of natural gas produces 2.41 × 104 MJ of electricity.
Calculate the percentage efficiency of the power station.
1 tonne = 1000 kg
Specific energy of natural gas used = 55.4 MJ kg−1 - 19N.3.sl.TZ0.12a: Suggest why a high-octane number fuel is preferable.
- 19N.3.hl.TZ0.20c: Suggest an advantage of the PEM fuel cell over the lead-acid battery for use in cars.
-
19N.3.sl.TZ0.14c:
Explain, including a suitable equation, why biofuels are considered to be carbon neutral.
- 19N.3.sl.TZ0.14d: State the type of reaction that occurs when ethanol reacts with vegetable oil to form biodiesel.
-
19N.3.hl.TZ0.20b(i):
Calculate the cell potential, Eθ, in V, using section 24 of the data booklet.
-
19N.3.sl.TZ0.13a(i):
State the nuclear equation for the fusion reaction.
-
19N.3.hl.TZ0.18a(i):
State the nuclear equation for the fusion reaction.
- 19N.3.hl.TZ0.18a(ii): Explain why fusion is an exothermic process.
-
19N.3.hl.TZ0.16a:
Discuss the data.
-
19N.3.hl.TZ0.18b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
Sub sections and their related questions
C.1 Energy sources
-
16N.3.sl.TZ0.11a:
(i) Calculate the specific energy of the lithium ion battery, in MJ kg−1, when 80.0 kg of fuel in the battery releases 1.58 × 107 J. Use section 1 of the data booklet.
(ii) The specific energy of gasoline is 46.0 MJ kg−1. Suggest why gasoline may be considered a better energy source than the lithium ion battery based on your answer to part (a) (i).
-
16N.3.sl.TZ0.11b:
(i) The energy density of gasoline is 34.3 MJ dm−3. Calculate the volume of gasoline, in dm3, that is equivalent to the energy in 80.0 kg of fuel in the lithium ion battery. Use section 1 of the data booklet.
(ii) The efficiency of energy transfer by this lithium ion battery is four times greater than that of gasoline. Determine the distance, in km, the car can travel on the lithium ion battery power alone if the gasoline-powered car uses 1.00 dm3 gasoline to travel 32.0 km.
-
17M.3.sl.TZ1.15c:
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
-
17M.3.sl.TZ1.15d:
Determine the specific energy, in kJg−1, and energy density, in kJcm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 gcm−3; Molar mass = 299 gmol−1;
Enthalpy of combustion = 12.0 MJmol−1.
-
17M.3.sl.TZ2.13a:
State one advantage and one disadvantage for each energy source in the table.
-
17M.3.sl.TZ2.13b.i:
Calculate the specific energy of hydrogen, stating its units. Refer to sections 1, 6 and 13 of the data booklet.
-
17M.3.sl.TZ2.13b.ii:
Hydrogen has a higher specific energy than petrol (gasoline) but is not used as a primary fuel source in cars. Discuss the disadvantages of using hydrogen.
-
17N.3.sl.TZ0.12a:
Calculate the specific energy of octane, C8H18, in kJ kg–1 using sections 1, 6 and 13 of the data booklet.
-
17N.3.sl.TZ0.12b:
A typical wood has a specific energy of 17 × 103 kJ kg–1. Comment on the usefulness of octane and wood for powering a moving vehicle, using your answer to (a).
If you did not work out an answer for (a), use 45 × 103 kJ kg–1 but this is not the correct answer.
-
17N.3.sl.TZ0.12c:
State the name of one renewable source of energy other than wood.
-
18M.3.sl.TZ1.10c.i:
Determine the specific energy and energy density of petrol (gasoline), using data from sections 1 and 13 of the data booklet. Assume petrol is pure octane, C8H18. Octane: molar mass = 114.26 g mol−1, density = 0.703 g cm−3.
-
18M.3.sl.TZ1.10c.ii:
Outline why the energy available from an engine will be less than these theoretical values.
-
18M.3.sl.TZ2.10a:
Outline two reasons why oil is one of the world’s significant energy sources.
-
18M.3.sl.TZ2.12a:
Calculate the thermal efficiency of a steam turbine supplied with steam at 540°C and using a river as the choice of sink at 23 °C.
-
18M.3.sl.TZ2.12b:
Power plants generating electricity by burning coal to boil water operate at approximately 35% efficiency.
State what this means and suggest why it is lower than the thermal efficiency.
-
18N.3.sl.TZ0.10b.i:
Calculate the specific energy, in kJ g−1, of methane.
-
18N.3.sl.TZ0.10b.ii:
Comment on the specific energies of hydrogen and methane.
- 18N.3.sl.TZ0.11a: Suggest another advantage and one disadvantage of solar energy.
-
18N.3.hl.TZ0.13b:
Comment on the specific energies of hydrogen and methane.
- 18N.3.hl.TZ0.14a: Suggest another advantage and one disadvantage of solar energy.
-
19M.3.hl.TZ1.15a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.hl.TZ1.15b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.hl.TZ1.15b(ii):
Hydroelectric power plants produced 16% of the world’s energy in 2015, down from 21% in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.hl.TZ2.14:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.hl.TZ2.15b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19M.3.sl.TZ1.11a:
Calculate the specific energy of methane, in MJ kg−1, using sections 1, 6 and 13 of the data booklet.
-
19M.3.sl.TZ1.11b(i):
Calculate the maximum electric energy output, in MJ, which can be obtained from burning 1.00 kg of methane by using your answer from (a).
-
19M.3.sl.TZ1.11b(ii):
Hydroelectric power plants produced 16 % of the world’s energy in 2015, down from 21 % in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
-
19M.3.sl.TZ2.9:
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
-
19M.3.sl.TZ2.10b:
Determine the specific energy, in kJ g−1, and energy density, in kJ cm−3, of hexane, C6H14. Give both answers to three significant figures.
Hexane: Mr = 86.2; ΔHc = −4163 kJ mol−1; density = 0.660 g cm−3
Specific energy:
Energy density:
-
19N.3.sl.TZ0.11a:
Discuss the data.
- 19N.3.hl.TZ0.16b: Outline what is meant by the degradation of energy.
-
19N.3.sl.TZ0.11b:
In a natural gas power station, 1.00 tonne of natural gas produces 2.41 × 104 MJ of electricity.
Calculate the percentage efficiency of the power station.
1 tonne = 1000 kg
Specific energy of natural gas used = 55.4 MJ kg−1 -
19N.3.hl.TZ0.16a:
Discuss the data.
-
20N.3.sl.TZ0.9a:
Calculate the energy released, in , from the complete combustion of of ethanol.
-
20N.3.hl.TZ0.11a:
Calculate the energy released, in , from the complete combustion of of ethanol.
C.2 Fossil fuels
- 16N.3.sl.TZ0.12a: Discuss how the octane number changes with the molecular structure of the alkanes.
- 16N.3.sl.TZ0.12b: Catalytic reforming and cracking reactions are used to produce more efficient fuels. Deduce the...
-
17M.3.sl.TZ1.16a:
State how these gases are produced, giving the appropriate equation(s).
-
17M.3.sl.TZ1.16b:
Outline how the carbon monoxide is then converted to a hydrocarbon fuel.
-
17M.3.sl.TZ2.14c.i:
Suggest an equation for the production of syngas from coal.
-
17M.3.sl.TZ2.14c.ii:
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
-
17M.3.sl.TZ2.14c.iii:
Suggest a reason why syngas may be considered a viable alternative to crude oil.
-
17N.3.sl.TZ0.13a:
“Knocking” in an automobile (car) engine can be prevented by increasing the octane number of the fuel. Explain, including an equation with structural formulas, how heptane, C7H16, could be chemically converted to increase its octane number.
-
17N.3.sl.TZ0.13b:
Many like to refer to our “carbon footprint”. Outline one difficulty in quantifying such a concept.
-
18M.3.sl.TZ1.10a:
Identify an element, other than carbon and hydrogen, found at significant concentrations in fossil fuels.
-
18M.3.sl.TZ1.10b:
Petroleum contains many hydrocarbons. Explain how these are separated by fractional distillation.
-
18M.3.sl.TZ2.10b.i:
Formulate an equation for the cracking of C16H34 into two products with eight carbon atoms each.
-
18M.3.sl.TZ2.10b.ii:
Identify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
-
18M.3.sl.TZ2.10c.i:
Outline how higher octane fuels help eliminate “knocking” in engines.
-
18M.3.sl.TZ2.10c.ii:
The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
-
18N.3.sl.TZ0.10a:
Formulate equation(s) for the conversion of coal and steam to methane.
-
18N.3.sl.TZ0.10c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
-
18N.3.hl.TZ0.13a:
Formulate equation(s) for the conversion of coal and steam to methane.
-
18N.3.hl.TZ0.13c:
Calculate the mass, in kg, of carbon dioxide produced by the complete combustion of 72.0 dm3 octane, C8H18.
Density of C8H18 = 703 g dm−3
C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g)
-
19M.3.hl.TZ1.15c(i):
Methane can also be obtained by fractional distillation of crude oil.
[Source: Image used with kind permission of science-resources.co.uk]
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.hl.TZ1.15c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.hl.TZ1.17a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.hl.TZ2.15a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.hl.TZ2.15c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
-
19M.3.sl.TZ1.11c(i):
Methane can also be obtained by fractional distillation of crude oil.
Draw a circle on the diagram to show where the methane fraction is withdrawn.
-
19M.3.sl.TZ1.11c(ii):
List the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
-
19M.3.sl.TZ1.13a:
Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
-
19M.3.sl.TZ1.13b:
Show that, for combustion of equal masses of fuel, ethanol (Mr = 46 g mol−1) has a lower carbon footprint than octane (Mr = 114 g mol−1).
-
19M.3.sl.TZ2.10a:
Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
-
19M.3.sl.TZ2.10c:
Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
- 19N.3.sl.TZ0.12a: Suggest why a high-octane number fuel is preferable.
- 19N.3.sl.TZ0.12b(i): Reforming reactions are used to increase the octane number of a hydrocarbon fuel. Suggest the...
- 19N.3.sl.TZ0.12b(ii): The 1H NMR spectrum of one of the products has four signals. The integration trace shows a ratio...
-
19N.3.sl.TZ0.14b:
Outline the evidence that relates global warming to increasing concentrations of greenhouse gases in the atmosphere.
- 20N.3.sl.TZ0.9b: State a class of organic compounds found in gasoline.
-
20N.3.sl.TZ0.9c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
-
20N.3.sl.TZ0.9d:
A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
- 20N.3.hl.TZ0.11b: State a class of organic compounds found in gasoline.
-
20N.3.hl.TZ0.11c:
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
- 20N.3.hl.TZ0.11d: A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture...
C.3 Nuclear fusion and fission
-
16N.3.sl.TZ0.15a:
(i) Explain why fusion, combining two smaller nuclei into a larger nucleus, releases vast amounts of energy. Use section 36 of the data booklet.
(ii) Outline one advantage of fusion as a source of energy.
-
16N.3.sl.TZ0.15b:
Radioactive phosphorus, 33P, has a half-life of 25.3 days.
(i) Calculate 33P decay constant λ and state its unit. Use section 1 of the data booklet.
(ii) Determine the fraction of the 33P sample remaining after 101.2 days.
-
17M.3.sl.TZ1.14a:
Outline how the spectra of light from stars can be used to detect the presence of carbon.
-
17M.3.sl.TZ1.14b.i:
Deduce the identity of X.
-
17M.3.sl.TZ1.14b.ii:
Outline why this reaction results in a release of energy.
-
17M.3.sl.TZ1.14c:
Nuclear fusion reactors are predicted to become an important source of electrical energy in the future. State two advantages of nuclear fusion over nuclear fission.
-
17M.3.sl.TZ2.12a.i:
One fusion reaction occurring in the sun is the fusion of deuterium, , with tritium, , to form helium, . State a nuclear equation for this reaction.
-
17M.3.sl.TZ2.12a.ii:
Explain why this fusion reaction releases energy by using section 36 of the data booklet.
-
17N.3.sl.TZ0.14a.i:
Compare and contrast fission and fusion in terms of binding energy and the types of nuclei involved.
-
17N.3.sl.TZ0.14a.ii:
Suggest two advantages that fusion has over fission.
-
17N.3.sl.TZ0.14b:
The amount of 228Ac in a sample decreases to one eighth of its original value in about 18 hours due to β-decay. Estimate the half-life of 228Ac.
-
18M.3.sl.TZ1.12a.ii:
Explain how 235U fission results in a chain reaction, including the concept of critical mass.
-
18M.3.sl.TZ1.12b:
Suggest one reason why there is opposition to the increased use of nuclear fission reactors.
-
18M.3.sl.TZ2.13a:
Compare and contrast the process of nuclear fusion with nuclear fission.
-
18M.3.sl.TZ2.13b:
Dubnium-261 has a half-life of 27 seconds and rutherfordium-261 has a half-life of 81 seconds.
Estimate what fraction of the dubnium-261 isotope remains in the same amount of time that of rutherfordium-261 decays.
- 18N.3.sl.TZ0.9a: Explain fusion reactions with reference to binding energy.
- 18N.3.sl.TZ0.9b.i: Outline why the term breeder is used for the reactors.
- 18N.3.sl.TZ0.9b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
-
18N.3.sl.TZ0.9c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
- 18N.3.hl.TZ0.12a: Explain fusion reactions with reference to binding energy.
- 18N.3.hl.TZ0.12b.i: Outline why the term breeder is used for the reactors.
- 18N.3.hl.TZ0.12b.ii: Deduce the fission reaction when 239Pu is bombarded with a neutron to produce 133Xe and 103Zr.
-
18N.3.hl.TZ0.12c:
Nuclear disasters release radioactive caesium into the atmosphere, which presents serious health risks.
Cs-137 has a half-life of 30 years.
Calculate the percentage of Cs-137 remaining in the atmosphere after 240 years.
-
19M.3.hl.TZ1.16a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.hl.TZ1.16a(ii):
Outline why the reaction releases energy.
-
19M.3.hl.TZ1.16b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.hl.TZ2.16a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.hl.TZ2.16a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.hl.TZ2.16a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.hl.TZ2.16b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
-
19M.3.sl.TZ1.12a(i):
Write the nuclear equation for this fission reaction.
-
19M.3.sl.TZ1.12a(ii):
Outline why the reaction releases energy.
-
19M.3.sl.TZ1.12b:
The critical mass for weapons-grade uranium can be as small as 15 kg. Outline what is meant by critical mass by referring to the equation in (a)(i).
-
19M.3.sl.TZ1.12c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for the mass of 89Kr to fall to 6.25 % of its initial value.
-
19M.3.sl.TZ2.11a(i):
Determine the other product of the fission reaction of plutonium-239.
-
19M.3.sl.TZ2.11a(ii):
Outline the concept of critical mass with respect to fission reactions.
-
19M.3.sl.TZ2.11a(iii):
Outline one advantage of allowing all countries access to the technology to generate electricity by nuclear fission.
-
19M.3.sl.TZ2.11b:
State one advantage of using fusion reactions rather than fission to generate electrical power.
-
19M.3.sl.TZ2.11c:
90Sr, a common product of fission, has a half-life of 28.8 years.
Determine the number of years for the activity of a sample of 90Sr to fall to one eighth () of its initial value.
-
19N.3.sl.TZ0.13a(i):
State the nuclear equation for the fusion reaction.
- 19N.3.sl.TZ0.13a(ii): Explain why fusion is an exothermic process.
-
19N.3.sl.TZ0.13b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
-
19N.3.hl.TZ0.18a(i):
State the nuclear equation for the fusion reaction.
- 19N.3.hl.TZ0.18a(ii): Explain why fusion is an exothermic process.
-
19N.3.hl.TZ0.18b:
Beryllium-8 is a radioactive isotope with a half-life of 6.70 × 10−17 s.
Calculate the mass of beryllium-8 remaining after 2.01 × 10−16 s from a sample initially containing 4.00 g of beryllium-8.
-
20N.3.sl.TZ0.10b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
- 20N.3.sl.TZ0.10c: Outline a health risk produced by exposure to radioactive decay.
-
20N.3.sl.TZ0.10d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
- 20N.3.sl.TZ0.10e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
-
20N.3.hl.TZ0.12b:
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
- 20N.3.hl.TZ0.12c: Outline a health risk produced by exposure to radioactive decay.
-
20N.3.hl.TZ0.12d:
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
- 20N.3.hl.TZ0.12e: Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by...
C.4 Solar energy
- 16N.3.sl.TZ0.14a: State the equation for the complete transesterification of the triglyceride given below with...
-
16N.3.sl.TZ0.14b:
Outline why the fuel produced by the reaction in (a) is more suitable for use in diesel engines than vegetable oils.
-
17M.3.sl.TZ1.15a:
State two reagents required to convert vegetable oil to biodiesel.
-
17M.3.sl.TZ1.15b:
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
-
17M.3.sl.TZ1.15c:
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
-
17M.3.sl.TZ1.15d:
Determine the specific energy, in kJg−1, and energy density, in kJcm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 gcm−3; Molar mass = 299 gmol−1;
Enthalpy of combustion = 12.0 MJmol−1.
-
17M.3.sl.TZ2.12b:
Coloured molecules absorb sunlight. Identify the bonding characteristics of such molecules.
- 17N.3.sl.TZ0.15a: State the structural feature of chlorophyll that enables it to absorb visible light.
- 17N.3.sl.TZ0.15b: Vegetable oils are too viscous for use as liquid fuels. Describe, using an equation, how a...
-
18M.3.hl.TZ1.13a:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
18M.3.hl.TZ1.13b:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.

-
18M.3.sl.TZ1.11a.i:
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
-
18M.3.sl.TZ1.11a.ii:
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
-
18M.3.sl.TZ1.11b:
Outline why biofuels are considered more environmentally friendly, even though they produce more carbon dioxide per kJ of energy than petroleum based fuels.
-
18M.3.sl.TZ2.14a:
Deduce the equation for the transesterification reaction of pentyl octanoate, C7H15COOC5H11, with methanol.
-
18M.3.sl.TZ2.14b:
Outline why the ester product of this reaction is a better diesel fuel than pentyl octanoate.
- 18N.3.sl.TZ0.11b: Light can be absorbed by chlorophyll and other pigments. Consider molecules A and B represented...
- 18N.3.sl.TZ0.11c.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
- 18N.3.sl.TZ0.11c.ii: Describe how vegetable oils can be converted to a more suitable fuel.
- 18N.3.hl.TZ0.14b.i: State a physical property of vegetable oils that makes them very difficult to use as fuel in...
- 18N.3.hl.TZ0.14b.ii: Describe how vegetable oils can be converted to a more suitable fuel.
-
19M.3.hl.TZ1.17c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.hl.TZ2.17:
This question is about biofuel.
Evaluate the use of biodiesel in place of diesel from crude oil.
-
19M.3.sl.TZ1.13c:
Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
-
19M.3.sl.TZ2.12a:
The structure of chlorophyll is given in section 35 of the data booklet.
State the feature of the chlorophyll molecule that enables it to absorb light in the visible spectrum.
-
19M.3.sl.TZ2.12b:
Evaluate the use of biodiesel in place of diesel from crude oil.
Strength:
Limitation:
-
19N.3.sl.TZ0.14a:
Write the equation for the complete combustion of ethanol.
-
19N.3.sl.TZ0.14c:
Explain, including a suitable equation, why biofuels are considered to be carbon neutral.
- 19N.3.sl.TZ0.14d: State the type of reaction that occurs when ethanol reacts with vegetable oil to form biodiesel.
-
20N.3.sl.TZ0.9d:
A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
- 20N.3.sl.TZ0.9e: Contrast the molecular structures of biodiesel and the vegetable oil from which it is formed.
- 20N.3.hl.TZ0.11d: A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture...
C.5 Environmental impact—global warming
-
16N.3.sl.TZ0.13a:
Explain the effect of the increasing concentration of atmospheric carbon dioxide on the acidity of oceans.
-
16N.3.sl.TZ0.13b:
(i) Describe the changes that occur at the molecular level when atmospheric carbon dioxide gas absorbs infrared radiation emitted from the Earth’s surface.
(ii) Other than changes to the acidity of oceans, suggest why the production of carbon dioxide is of greater concern than the production of water vapour.
-
17M.3.sl.TZ1.17a:
Suggest why it is only in recent years that specific predictions of the future effects of fossil fuel combustion have been made.
-
17M.3.sl.TZ1.17b:
Carbon dioxide has two different bond stretching modes illustrated below.

Predict, with an explanation, whether these stretching modes will absorb infrared radiation.
-
17M.3.sl.TZ1.17c:
Outline, giving the appropriate equation(s), how increasing levels of carbon dioxide will affect the pH of the oceans.
-
17M.3.sl.TZ1.17d:
Many combustion processes also release particulate matter into the atmosphere. Suggest, giving your reason, how this might affect the temperature of the Earth’s surface.
-
17M.3.sl.TZ2.14a:
Identify which region, A or B, corresponds to each type of radiation by completing the table.
-
17M.3.sl.TZ2.14b.i:
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
-
17M.3.sl.TZ2.14b.ii:
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
-
17N.3.sl.TZ0.13c:
Climate change or global warming is a consequence of increased levels of carbon dioxide in the atmosphere. Explain how the greenhouse effect warms the surface of the earth.
- 17N.3.sl.TZ0.13d: Outline how water and carbon dioxide absorb infrared radiation.
-
18M.3.sl.TZ1.9a:
Identify one naturally occurring greenhouse gas, other than carbon dioxide or water vapour, and its natural source.
-
18M.3.sl.TZ1.9b:
Formulate an equation that shows how aqueous carbon dioxide produces hydrogen ions, H+(aq).
-
18M.3.sl.TZ1.9c:
The concentrations of oxygen and nitrogen in the atmosphere are much greater than those of greenhouse gases. Outline why these gases do not absorb infrared radiation.
-
18M.3.sl.TZ2.11a:
Explain the molecular mechanism by which carbon dioxide acts as a greenhouse gas.
-
18M.3.sl.TZ2.11b:
Discuss the significance of two greenhouse gases, other than carbon dioxide, in causing global warming or climate change.
- 18N.3.sl.TZ0.11d: Contrast the importance of carbon dioxide and methane as greenhouse gases.
-
18N.3.sl.TZ0.11e:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
- 18N.3.hl.TZ0.14c: Contrast the importance of carbon dioxide and methane as greenhouse gases.
-
18N.3.hl.TZ0.14d:
Explain, using an equation, the effect of increased carbon dioxide in the atmosphere on the pH of lake water.
-
19M.3.hl.TZ1.15d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.hl.TZ1.15d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.hl.TZ2.18a:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.hl.TZ2.18b:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
19M.3.sl.TZ1.11d(i):
Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
-
19M.3.sl.TZ1.11d(ii):
Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
-
19M.3.sl.TZ2.13a:
State one greenhouse gas, other than carbon dioxide.
-
19M.3.sl.TZ2.13b:
Describe the effect of infrared (IR) radiation on carbon dioxide molecules.
-
19M.3.sl.TZ2.13c:
Outline one approach to controlling industrial emissions of carbon dioxide.
-
20N.3.sl.TZ0.9f(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
-
20N.3.sl.TZ0.9f(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
-
20N.3.hl.TZ0.11e(i):
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
-
20N.3.hl.TZ0.11e(ii):
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
C.6 Electrochemistry, rechargeable batteries and fuel cells (HL only)
-
16N.3.hl.TZ0.21a:
The Geobacter species of bacteria can be used in microbial fuel cells to oxidise aqueous ethanoate ions,
CH3COO−(aq), to carbon dioxide gas.State the half-equations for the reactions at both electrodes.
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
-
17M.3.hl.TZ1.22a:
Deduce half-equations for the reactions at the two electrodes and hence the equation for the overall reaction.
-
17M.3.hl.TZ1.22b.i:
Suggest a way in which they are similar.
-
17M.3.hl.TZ1.22b.ii:
Outline the difference between primary and rechargeable cells.
-
17M.3.hl.TZ1.22c:
Identify one factor that affects the voltage of a cell and a different factor that affects the current it can deliver.
-
17M.3.hl.TZ2.17c.i:
Deduce the half-cell equations occurring at each electrode during discharge.
-
17M.3.hl.TZ2.17c.ii:
Outline the function of the proton-exchange membrane (PEM) in the fuel cell.
-
17M.3.hl.TZ2.17c.iii:
Explain how the flow of ions allows for the operation of the fuel cell.
-
17N.3.hl.TZ0.20a:
Deduce the half-equations and the overall equation for the reactions taking place in a direct methanol fuel cell (DMFC) under acidic conditions.
-
17N.3.hl.TZ0.20b:
Outline one advantage and one disadvantage of the methanol cell (DMFC) compared with a hydrogen-oxygen fuel cell.
-
18M.3.hl.TZ1.14a.i:
Complete the half-equations on the diagram and identify the species moving between the electrodes.

-
18M.3.hl.TZ1.14a.ii:
State the factor that limits the maximum current that can be drawn from this cell and how electrodes are designed to maximize the current.
-
18M.3.hl.TZ2.13c:
Fuel cells have a higher thermodynamic efficiency than octane. The following table gives some information on a direct methanol fuel cell.

Determine the thermodynamic efficiency of a methanol fuel cell operating at 0.576 V.
Use sections 1 and 2 of the data booklet.
- 18N.3.hl.TZ0.15a: Outline how a rechargeable battery differs from a primary cell.
-
18N.3.hl.TZ0.15b:
Formulate half-equations for the reactions at the anode (negative electrode) and cathode (positive electrode) during discharge of a lithium-ion battery.
-
18N.3.hl.TZ0.15c:
A voltaic cell consists of a nickel electrode in 1.0 mol dm−3 Ni2+ (aq) solution and a cadmium electrode in a Cd2+ (aq) solution of unknown concentration.
Cd (s) + Ni2+ (aq) → Cd2+ (aq) + Ni (s) EΘcell = 0.14 V
Determine the concentration of the Cd2+ (aq) solution if the cell voltage, E, is 0.19 V at 298 K. Use section 1 of the data booklet.
-
19M.3.hl.TZ1.17b(i):
Ethanol can be used in a direct-ethanol fuel cell (DEFC) as illustrated by the flow chart.
Deduce the half-equations occurring at electrodes A and B.
Electrode A:
Electrode B:
-
19M.3.hl.TZ1.17b(ii):
State the name and function of X in the diagram in (b)(i).
Name:
Function:
-
19M.3.hl.TZ1.17b(iii):
Outline why aqueous ethanol, rather than pure ethanol, is used in a DEFC.
-
19M.3.hl.TZ2.19a:
Outline how a microbial fuel cell produces an electric current from glucose.
C6H12O6 (aq) + 6O2 (g) → 6CO2 (g) + 6H2O (l)
-
19M.3.hl.TZ2.19b:
The cell potential for the spontaneous reaction when standard magnesium and silver half-cells are connected is +3.17 V.
Determine the cell potential at 298 K when:
[Mg2+] = 0.0500 mol dm−3
[Ag+] = 0.100 mol dm−3Use sections 1 and 2 of the data booklet.
- 19M.3.hl.TZ2.19c: Outline one difference between a primary and a secondary cell.
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
-
19N.3.hl.TZ0.20b(i):
Calculate the cell potential, Eθ, in V, using section 24 of the data booklet.
-
19N.3.hl.TZ0.20b(ii):
Suggest how PEM fuel cells can be used to produce a larger voltage than that calculated in (b)(i).
- 19N.3.hl.TZ0.20c: Suggest an advantage of the PEM fuel cell over the lead-acid battery for use in cars.
C.7 Nuclear fusion and nuclear fission (HL only)
-
17M.3.hl.TZ1.18b.ii:
The mass of X is 8.005305 amu and that of is 4.002603 amu. Determine the energy produced, in J, when one atom of is formed in this reaction. Use section 2 of the data booklet.
-
17M.3.hl.TZ2.16a.iii:
Calculate the energy released, in MeV, in this reaction, using section 36 of the data booklet.
-
17N.3.hl.TZ0.18c.i:
Calculate the loss in mass, in kg, and the energy released, in J, when 0.00100 mol of 228Ac decays, each atom losing an electron. Use section 2 of the data booklet and E = mc2.
228Ac → + 228Th
-
17N.3.hl.TZ0.18c.ii:
Determine the energy released, in J, by 0.00100 mol of 228Ac over the course of 18 hours.
- 17N.3.hl.TZ0.18d: Outline how nuclear ionising radiation can damage DNA and enzymes in living cells.
-
18M.3.hl.TZ1.14b.ii:
Explain how the proportion of 235U in natural uranium is increased.
-
18M.3.hl.TZ2.16c.i:
Calculate the relative rate of effusion of 235UF6(g) to 238UF6(g) using sections 1 and 6 of the data booklet.
-
18M.3.hl.TZ2.16c.ii:
Explain, based on molecular structure and bonding, why diffusion or centrifuging can be used for enrichment of UF6 but not UO2.
-
18N.3.hl.TZ0.12d.i:
Deduce a Lewis (electron dot) structure of the superoxide, O2–, free radical.
- 18N.3.hl.TZ0.12d.ii: Explain why free radicals are harmful to living cells.
-
19M.3.hl.TZ1.16a(iii):
The masses of the particles involved in this fission reaction are shown below.
Mass of neutron = 1.00867 amu
Mass of U-235 nucleus = 234.99346 amu
Mass of Ba-144 nucleus = 143.89223 amu
Mass of Kr-89 nucleus = 88.89788 amuDetermine the energy released, in J, when one uranium-235 nucleus undergoes fission. Use this data and information from sections 1 and 2 of the data booklet.
-
19M.3.hl.TZ1.16c:
The daughter product, 89Kr, has a half-life of 3.15 min.
Calculate the time required, in minutes, for its radioactivity to fall to 10% of its initial value, using section 1 of the data booklet.
-
19M.3.hl.TZ2.16c:
Outline how the energy of a fission reaction can be calculated.
-
19M.3.hl.TZ2.16d:
Calculate the half-life of an isotope whose mass falls from 5.0 × 10−5 g to 4.0 × 10−5 g in 31.4 s, using section 1 of the data booklet.
-
19N.3.hl.TZ0.18a(iii):
Calculate the heat energy released, in J, by the fusion reaction producing one atom of carbon-12. Use section 2 of the data booklet and E = mc2.
-
20N.3.hl.TZ0.11e(iv):
Determine the relative rate of effusion of methane () to carbon dioxide (), under the same conditions of temperature and pressure. Use section 1 of the data booklet.
-
20N.3.hl.TZ0.12f:
Determine the nuclear binding energy, in , of using sections 2 and 4 of the data booklet.
The mass of the nucleus is .
C.8 Photovoltaic and dye-sensitized solar cells (HL only)
-
16N.3.hl.TZ0.21c:
Dye-sensitized solar cells (DSSC) convert solar energy into electrical energy.
(i) Describe how a DSSC converts sunlight into electrical energy.
(ii) Explain the role of the electrolyte solution containing iodide ions, I−, and triiodide ions, I3−, in the DSSC.
-
17M.3.hl.TZ1.19a:
Identify two ways in which the structure of the dye shown resembles the chlorophyll molecule. Use section 35 of the data booklet.
-
17M.3.hl.TZ1.19b:
Both photosynthesis and the Grätzel cell use energy from sunlight to bring about reduction. Deduce an equation for the reduction reaction in the electrolyte of a Grätzel cell.
-
17M.3.hl.TZ2.18a.ii:
The structures of 11-cis-retinal and β-carotene are given in section 35 of the data booklet. Suggest a possible wavelength of light absorbed by each molecule using section 3 of the data booklet.
-
17M.3.hl.TZ2.19a:
Contrast how absorption of photons and charge separation occur in each device.
-
17M.3.hl.TZ2.19b:
Suggest one advantage a DSSC has over a silicon based photovoltaic cell.
-
17N.3.hl.TZ0.19b:
The natural absorption of light by chlorophyll has been copied by those developing dye-sensitized solar cells (DSSCs). Outline how a DSSC works.
-
18M.3.hl.TZ1.15a:
Early photovoltaic cells were based on silicon containing traces of other elements. State the type of semiconductor produced by doping silicon with indium, In, giving a reason that refers to its electronic structure.
-
18M.3.hl.TZ1.15b:
Dye-sensitized solar cells, DSSCs, use a dye to absorb the sunlight. State two advantages that DSSCs have over traditional silicon based photovoltaic cells.
-
18M.3.hl.TZ1.15c:
The structure of two dyes used in DSSCs are shown.

Predict, giving a reason, which dye will absorb light of longer wavelength.
-
18M.3.hl.TZ2.18a:
Draw the Lewis (electron dot) structure for an appropriate doping element in the box in the centre identifying the type of semiconductor formed.

-
18M.3.hl.TZ2.18b.i:
State the feature of the molecules responsible for the absorption of light.
-
18M.3.hl.TZ2.18b.ii:
Outline why complex B absorbs light of longer wavelength than complex A.
- 18N.3.hl.TZ0.15d.i: Identify the structural feature of the dye that allows the conversion of solar energy into...
- 18N.3.hl.TZ0.15d.ii: Outline the effect of sunlight on the dye in the solar cell.
- 18N.3.hl.TZ0.15d.iii: State the purpose of TiO2.
-
18N.3.hl.TZ0.15d.iv:
Deduce the reduction half-equation at the cathode.
-
19M.3.hl.TZ1.18a:
Some solar cells use photovoltaic semi-conductors. Compare, giving reasons, the electrical conductivity of metals and semi-conductors as temperature increases.
-
19M.3.hl.TZ1.18b:
Suggest one advantage of a dye-sensitized solar cell (DSSC) over a silicon based photovoltaic cell.
-
19M.3.hl.TZ2.20a:
Sketch graphs to show the general effect of increasing temperature on the electrical conductivity of semiconductors and metals on the axes below.
-
19M.3.hl.TZ2.20b:
Explain the function of dyes in a dye-sensitized solar cell (DSSC).
- 19N.3.hl.TZ0.20d(i): Outline the functions of the dye, TiO2 and the electrolyte in the operation of the...
- 19N.3.hl.TZ0.20d(ii): Suggest an advantage of the DSSC over silicon-based photovoltaic cells.
-
20N.3.hl.TZ0.14a:
Doping of silicon increases the conductivity in semiconductors.
Describe the doping in p-type and n-type semiconductors.
- 20N.3.hl.TZ0.14b: Doping of silicon increases the conductivity in semiconductors. Explain how doping improves the...
