| Date | November 2020 | Marks available | 1 | Reference code | 20N.3.hl.TZ0.6 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | State | Question number | 6 | Adapted from | N/A |
Question
Proteins are polymers of amino acids.
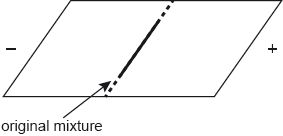
A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
Proteins are polymers of amino acids.
Sketch and label two oxygen dissociation curves, one for adult hemoglobin and one for foetal hemoglobin.
Proteins are polymers of amino acids.
Explain why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin.
Markscheme
✔
Accept any value within the range “”.
Ile AND larger Rf ✔
more soluble in non-polar solvent «mobile phase»
OR
not as attracted to polar «stationary» phase ✔
Only award M2 if Ile is identified in M1.
hydrogen/ bonding «between amido hydrogen and carboxyl oxygen atoms» ✔
both curves sigmoidal shape AND starting at zero ✔
foetal hemoglobin showing greater affinity/steeper/higher gradient ✔
Do not penalize if convergence is not approached for M1.
Both curves must be labelled to score M2.
Any two of:
contains two gamma/ units «instead of two beta/ units found in adults»
OR
differs in amino acid sequence «from the two beta// units found in adults» ✔
less sensitive to inhibitors/2,3-BPG/DPG ✔
receives from «partly deoxygenated» blood so can work at low ✔
low in foetal blood increases affinity for ✔
hemoglobin concentration in foetal blood greater than in the mother ✔
Examiners report
It was surprising that more did not manage to determine the Rf value of A1 within the acceptable range of "0.67 to 0.73". An out of range value of 0.75 was frequently seen.
Few candidates recognised that A1 was Ile so no marks were scored. Even of those candidates that did identify A1 as IIe, many did not mention larger Rf and therefore M1 was lost. There appeared to be in general, poor understanding of the basic principles of paper chromatography.
A majority stated that hydrogen bonding is the bonding responsible for the alpha-helix in the secondary structure.
The better candidates scored both marks in this question on oxygen dissociation curves. Many scored M2 for showing foetal hemoglobin having a higher gradient. Frequently sigmoidal curves were not drawn which lost M1 and often both curves were not explicitly labelled which was necessary to score M2.
This question required an explanation as to why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin. This question was poorly answered and only the stronger candidates managed to score both marks, usually by stating that foetal hemoglobin contains two gamma units instead of the two beta units found in adult hemoglobin, required for M1 and often by stating that there is less sensitivity to inhibitors for M2.