DP Chemistry Questionbank

B: Biochemistry
| Path: |
Description
[N/A]Directly related questions
- 16N.3.sl.TZ0.8a: Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed...
-
16N.3.sl.TZ0.9b:
The structures of two molecules, X and Y, are shown below.
(i) Justify why both these molecules are carbohydrates.
(ii) Distinguish between these molecules in terms of their functional groups.
-
16N.3.sl.TZ0.10b:
A mixture of amino acids is separated by gel electrophoresis at pH 6.0. The amino acids are then stained with ninhydrin.
(i) On the diagram below draw the relative positions of the following amino acids at the end of the process: Val, Asp, Lys and Thr.
(ii) Suggest why glycine and isoleucine separate slightly at pH 6.5.
-
16N.3.hl.TZ0.13c:
Amino acids act as buffers in solution. In aspartic acid, the side chain (R group) carboxyl has pKa = 4.0. Determine the percentage of the side chain carboxyl that will be ionized (–COO–) in a solution of aspartic acid with pH = 3.0. Use section 1 of the data booklet.
-
16N.3.sl.TZ0.10d:
The fibrous protein keratin has a secondary structure with a helical arrangement.
(i) State the type of interaction responsible for holding the protein in this arrangement.
(ii) Identify the functional groups responsible for these interactions.
-
16N.3.sl.TZ0.9c:
Amylose is an unbranched polysaccharide composed of repeating units of glucose.
(i) Draw the structure of the repeating unit of amylose. Use section 34 of the data booklet.
(ii) Amylose is a major component of starch. Corn starch can be used to make replacements for plastics derived from oil, especially for packaging. Discuss one potential advantage and one disadvantage of this use of starch.
-
16N.3.hl.TZ0.16c:
A student investigated the ability of anthocyanins to act as pH indicators. He extracted juice from blackberries and used a UV-vis spectrophotometer to produce absorption spectra at different pH values. His results are shown below.
Deduce the colour of the juice at each pH, giving your reasoning. Use section 17 of the data booklet.
-
16N.3.sl.TZ0.8b:
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
-
16N.3.hl.TZ0.15b:
In 2010, scientists claimed that they had discovered a species of bacteria capable of incorporating arsenic in place of phosphorus into the bacterial DNA. This claim has since proved controversial. Suggest one technique or evidence that might help support the claim.
- 16N.3.sl.TZ0.9a: State the raw materials and source of energy used in the process described above.
- 16N.3.sl.TZ0.10c: Determine the number of different tripeptides that can be made from twenty different amino acids.
-
16N.3.hl.TZ0.14b:
(i) Outline what is meant by product inhibition as it applies to hexokinase.
(ii) Product inhibition of hexokinase does not affect its Km value. Using this information, deduce the type of binding site that the inhibitor attaches to.
- 16N.3.hl.TZ0.15a: State the name of the component of DNA responsible for the migration of its fragments to the...
- 16N.3.hl.TZ0.16a: Outline why this molecule absorbs visible light.
-
16N.3.hl.TZ0.14a:
(i) Estimate the Km values of the two enzymes.
(ii) Suggest, with a reason, which enzyme will be more responsive to changes in the concentration of glucose in the blood.
- 16N.3.hl.TZ0.16b: With reference to its chemical structure, outline whether this pigment is found in aqueous...
-
20N.3.sl.TZ0.5b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
-
20N.3.sl.TZ0.6a:
Deduce the products of the hydrolysis of a non-substituted phospholipid, where and represent long alkyl chains.
- 20N.3.sl.TZ0.7a: The diverse functions of biological molecules depend on their structure and shape. Classify...
-
20N.3.sl.TZ0.7b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
-
20N.3.sl.TZ0.8a:
Calculate the BMF if a shark consumes mackerel in one year. Each mackerel weighs on average. The per body weight. Assume chemical remains in the shark’s body for two years.
-
20N.3.sl.TZ0.7b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Sucrose is a disaccharide formed in the reaction of glucose with fructose.
Identify the reaction type and the newly formed functional group that joins the monosaccharide units in the product.
-
20N.3.sl.TZ0.6c:
Phospholipids help maintain cellular environments while fatty acid lipids have important roles in energy storage and electrical insulation. Discuss the structural properties of saturated fats needed for these roles.
- 20N.3.sl.TZ0.8b: Suggest, with a reason, if fat-soluble or water-soluble xenobiotics would have a larger BMF.
-
20N.3.sl.TZ0.6b(i):
A representation of a phospholipid bilayer cell membrane is shown:
© International Baccalaureate Organization 2020.
Identify the components of the phospholipid labelled A and B.
-
20N.3.sl.TZ0.5a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.sl.TZ0.5c:
Proteins are polymers of amino acids.
Describe how the tertiary structure differs from the quaternary structure in hemoglobin.
-
20N.3.sl.TZ0.5a(i):
Proteins are polymers of amino acids. A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
-
20N.3.hl.TZ0.6a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.hl.TZ0.6b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
-
20N.3.hl.TZ0.10b(ii):
Outline the significance of the value of the Michaelis constant, .
- 20N.3.hl.TZ0.10a: Identify the type of inhibition shown in the graph.
-
20N.3.hl.TZ0.8b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Draw the nitrogenous base that is paired with guanine in DNA, showing the hydrogen bonds between the bases. Use section 34 of the data booklet.
-
20N.3.hl.TZ0.6c(ii):
Proteins are polymers of amino acids.
Explain why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin.
-
20N.3.hl.TZ0.6a(i):
Proteins are polymers of amino acids.
A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
- 20N.3.hl.TZ0.6c(i): Proteins are polymers of amino acids. Sketch and label two oxygen dissociation curves, one for...
-
20N.3.hl.TZ0.8b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
- 20N.3.hl.TZ0.8c: The diverse functions of biological molecules depend on their structure and shape. Retinal is...
- 20N.3.hl.TZ0.8a: The diverse functions of biological molecules depend on their structure and shape. Classify...
-
20N.3.hl.TZ0.10b(i):
Determine the value of and in the absence and presence of the inhibitor.
-
17M.3.sl.TZ1.11a:
List the building blocks of triglycerides and carbohydrates.
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ1.11b.ii:
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
-
17M.3.sl.TZ1.11c:
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16. -
17M.3.sl.TZ1.12a.i:
Deduce the straight chain structure of ribose from its ring structure drawn in section 34 of the data booklet.
-
17M.3.sl.TZ1.12a.ii:
Using the partial structure given, complete the structural formula of the molecule formed from the condensation of two cyclic -glucose molecules.
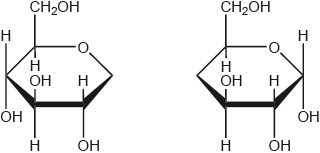
-
17M.3.sl.TZ1.12b:
Constructing models that allow visualizations of the stereochemistry of carbohydrates is essential to understand their structural roles in cells.
Describe how Haworth projections help focus on the position of attached groups.
-
17M.3.sl.TZ1.12c.i:
State one advantage of starch based polymers besides being biodegradable.
-
17M.3.sl.TZ1.12c.ii:
Biodegradable boxes made from polylactic acid, PLA, disintegrate when exposed to water.
State the formula of the product formed when water reacts with PLA.
-
17M.3.sl.TZ1.13a:
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
-
17M.3.sl.TZ1.13b.i:
Identify the name of the amino acid that does not move under the influence of the applied voltage.
-
17M.3.sl.TZ1.13b.ii:
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
-
17M.3.sl.TZ1.13c:
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
-
17M.3.sl.TZ1.13d:
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
-
17M.3.sl.TZ1.13e:
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
-
17M.3.sl.TZ1.13f:
Outline how lead ions could be removed from an individual suffering from lead poisoning.
-
17M.3.hl.TZ1.15a:
Deduce the pH range in which glycine is an effective buffer in basic solution.
-
17M.3.hl.TZ1.15b:
Enzymes are biological catalysts.
The data shows the effect of substrate concentration, [S], on the rate, v, of an enzyme-catalysed reaction.
Determine the value of the Michaelis constant (Km) from the data. A graph is not required.
-
17M.3.hl.TZ1.15c:
Outline the action of a non-competitive inhibitor on the enzyme-catalysed reaction.
-
17M.3.hl.TZ1.15d:
The sequence of nitrogenous bases in DNA determines hereditary characteristics.
Calculate the mole percentages of cytosine, guanine and thymine in a double helical DNA structure if it contains 17% adenine by mole.
-
17M.3.hl.TZ1.16a:
Explain the shape of the curve at low oxygen partial pressure up to about 5 kPa.
-
17M.3.hl.TZ1.16b.i:
Sketch a graph on the axes above to show the effect of decreasing pH on the binding of oxygen to hemoglobin (the Bohr Effect).
-
17M.3.hl.TZ1.16b.ii:
Outline the effect of decreasing pH on the oxygen saturation of hemoglobin.
-
17M.3.hl.TZ1.17:
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
-
17M.3.sl.TZ2.7a:
Deduce the structural formula of the dipeptide Cys-Lys.
-
17M.3.sl.TZ2.7b:
Identify the type of bond between two cysteine residues in the tertiary structure of a protein.
-
17M.3.sl.TZ2.7c:
Deduce the structural formula of the predominant form of cysteine at pH 1.0.
-
17M.3.sl.TZ2.7d:
A mixture of the three amino acids, cysteine, glutamine and lysine, was placed in the centre of a square plate covered in polyacrylamide gel. The gel was saturated with a buffer solution of pH 6.0. Electrodes were connected to opposite sides of the gel and a potential difference was applied.
Sketch lines on the diagram to show the relative positions of the three amino acids after electrophoresis.
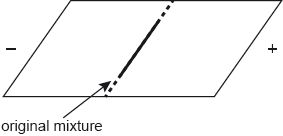
-
17M.3.sl.TZ2.8a:
Explain which one of these fatty acids has the highest boiling point.
-
17M.3.sl.TZ2.8b:
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 moldm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
-
17M.3.sl.TZ2.9a:
Glycerol is one product of the reaction. Identify the two other organic products.
-
17M.3.sl.TZ2.9b:
Identify the type of reaction which occurs.
-
17M.3.sl.TZ2.10a:
Identify the functional groups which are present in only one structure of glucose.
-
17M.3.sl.TZ2.10b:
Sucrose is a disaccharide formed from -glucose and β-fructose.
Deduce the structural formula of sucrose.
-
17M.3.sl.TZ2.10c:
Starch is a constituent of many plastics. Suggest one reason for including starch in plastics.
-
17M.3.sl.TZ2.10d:
Suggest one of the challenges scientists face when scaling up the synthesis of a new compound.
-
17M.3.sl.TZ2.11:
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
-
17M.3.hl.TZ2.8c.i:
An aqueous buffer solution contains both the zwitterion and the anionic forms of alanine. Draw the zwitterion of alanine.
-
17M.3.hl.TZ2.8c.ii:
Calculate the pH of a buffer solution which contains 0.700 mol dm–3 of the zwitterion and 0.500 mol dm–3 of the anionic form of alanine.
Alanine pKa = 9.87.
-
17M.3.hl.TZ2.12a:
Identify the structural feature which enables rhodopsin to absorb visible light.
-
17M.3.hl.TZ2.12b:
Outline the change that occurs in the retinal residue during the absorption of visible light.
-
17M.3.hl.TZ2.13a:
Determine the value of the Michaelis constant, Km, including units, from the graph.
-
17M.3.hl.TZ2.13b:
Sketch a second graph on the same axes to show how the reaction rate varies when a competitive inhibitor is present.
-
17M.3.hl.TZ2.13c:
Outline the significance of the value of Km.
-
17M.3.hl.TZ2.14a:
Explain the shape of the curve from 0 to X kPa.
-
17M.3.hl.TZ2.14b:
Explain why carbon monoxide is toxic to humans.
-
17M.3.hl.TZ2.15a:
Outline how its structure allows it to be negatively charged in the body.
-
17M.3.hl.TZ2.15b:
Deduce the nucleotide sequence of a complementary strand of a fragment of DNA with the nucleotide sequence –GACGGATCA–.
- 17N.3.sl.TZ0.8a.ii: The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per...
-
17N.3.sl.TZ0.8c:
Outline the importance of linoleic acid for human health.
-
17N.3.hl.TZ0.11a:
Determine the value of the Michaelis constant, Km, by annotating the graph.
-
17N.3.hl.TZ0.13:
The stability of DNA is due to interactions of its hydrophilic and hydrophobic components.
Outline the interactions of the phosphate groups in DNA with water and with surrounding proteins (histones).
-
17N.3.sl.TZ0.9c:
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
-
17N.3.hl.TZ0.11b.i:
The malonate ion acts as an inhibitor for the enzyme.
Suggest, on the molecular level, how the malonate ion is able to inhibit the enzyme.
-
17N.3.hl.TZ0.11b.ii:
Draw a curve on the graph above showing the effect of the presence of the malonate ion inhibitor on the rate of reaction.
-
17N.3.hl.TZ0.14a:
State the half-equation for the reduction of molecular oxygen to water in acidic conditions.
- 17N.3.hl.TZ0.14b: Outline the change in oxidation state of the iron ions in heme groups that occurs when molecular...
-
17N.3.hl.TZ0.15b:
Retinal is the key molecule involved in vision. Explain the roles of cis and trans-retinal in vision and how the isomers are formed in the visual cycle.
-
17N.3.sl.TZ0.10a:
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
- 17N.3.sl.TZ0.9b: Draw the structure of galactose on the skeleton provided.
- 17N.3.sl.TZ0.10b: State one function of vitamin D in the body.
- 17N.3.sl.TZ0.11: Enzyme activity depends on many factors. Explain how pH change causes loss of activity of an enzyme.
-
17N.3.sl.TZ0.9a:
Describe what is meant by a condensation reaction.
-
17N.3.sl.TZ0.8b.ii:
Calculate the volume of iodine solution used to reach the end-point.
- 17N.3.sl.TZ0.8b.i: State the type of reaction occurring during the titration.
-
18M.3.hl.TZ1.10a:
Outline why anthocyanins are coloured.
-
18M.3.hl.TZ1.10b:
Explain why the blue colour of a quinoidal base changes to the red colour of a flavylium cation as pH decreases.
-
18M.3.hl.TZ2.11a:
Hemoglobin’s oxygen dissociation curve is shown at a given temperature. Sketch the curve on the graph at a higher temperature.
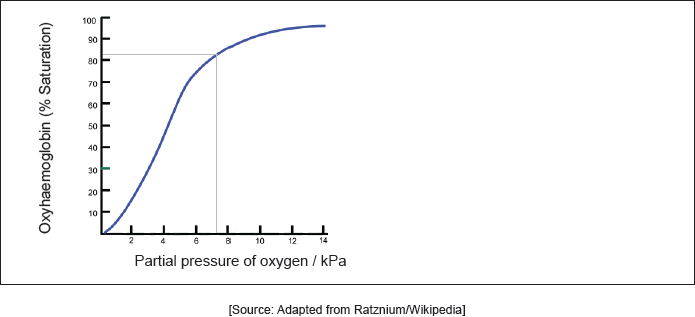
-
18M.3.hl.TZ1.6d:
Describe how DNA determines the primary structure of a protein such as insulin.
-
18M.3.hl.TZ1.8b:
Outline why cellulose fibres are strong.
-
18M.3.hl.TZ2.11b:
Outline two differences between normal hemoglobin and foetal hemoglobin.
-
18M.3.hl.TZ1.9b:
Outline the significance of the value of the Michaelis constant, Km.
-
18M.3.hl.TZ2.12:
DNA is a biopolymer made up of nucleotides. List two components of a nucleotide.
-
18M.3.hl.TZ2.8d:
Calculate the pH of a buffer system with a concentration of 1.25 × 10−3 mol dm−3 carbonic acid and 2.50 × 10−2 mol dm−3 sodium hydrogen carbonate. Use section 1 of the data booklet.
pKa (carbonic acid) = 6.36
-
18M.3.hl.TZ1.9a:
Explain with reference to the binding site on the enzyme how a non-competitive inhibitor lowers the value of Vmax.
-
18M.3.hl.TZ2.10b:
Explain how the structure of vitamin A is important to vision using section 35 of the data booklet.
-
18M.3.hl.TZ2.8c:
Draw the structures of the main form of glycine in buffer solutions of pH 1.0 and 6.0.
The pKa of glycine is 2.34.
-
18M.3.sl.TZ1.7a.ii:
State one factor that increases the rate at which saturated lipids become rancid.
-
18M.3.sl.TZ1.7c.iv:
Plastics are another source of marine pollution. Outline one way in which plastics can be made more biodegradable.
-
18M.3.sl.TZ1.8b:
A person with diabetes suffering very low blood sugar (hypoglycaemia) may be advised to consume glucose immediately and then eat a small amount of starchy food such as a sandwich. Explain this advice in terms of the properties of glucose and starch.
-
18M.3.sl.TZ1.7c.i:
Fish oil is an excellent dietary source of omega-3 fatty acids. Outline one impact on health of consuming omega-3 fatty acids.
-
18M.3.sl.TZ1.6c.i:
State the name of the process used to break down the insulin protein into its constituent amino acids.
-
18M.3.sl.TZ1.7b:
Butter contains varying proportions of oleic, myristic, palmitic and stearic acids. Explain in terms of their structures why stearic acid has a higher melting point than oleic acid, using section 34 of the data booklet.
-
18M.3.sl.TZ1.7c.iii:
Explain why sharks and swordfish sometimes contain high concentrations of mercury and polychlorinated biphenyls (PCBs).
-
18M.3.sl.TZ1.8a:
State the specific type of linkage formed between α-glucose fragments in both maltose and amylose.
-
18M.3.sl.TZ1.6a:
Draw the structural formula of a dipeptide containing the residues of valine, Val, and asparagine, Asn, using section 33 of the data booklet.
-
18M.3.sl.TZ1.6b:
Deduce the strongest intermolecular forces that would occur between the following amino acid residues in a protein chain.
-
18M.3.sl.TZ1.6c.ii:
Outline how the amino acids may be identified from a paper chromatogram.
-
18M.3.sl.TZ1.7a.i:
Identify the type of rancidity occurring in saturated lipids and the structural feature that causes it.
-
18M.3.sl.TZ1.7c.ii:
Predict the solubility of retinol (vitamin A) in body fat, giving a reason. Use section 35 of the data booklet.
-
18M.3.sl.TZ2.6a:
Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
-
18M.3.sl.TZ2.6c:
Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
-
18M.3.sl.TZ2.6d:
Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
-
18M.3.sl.TZ2.8:
Green Chemistry reduces the production of hazardous materials and chemical waste.
Outline two specific examples or technological processes of how Green Chemistry has accomplished this environmental impact.
-
18M.3.sl.TZ2.9:
Explain the solubility of vitamins A and C using section 35 of the data booklet.
-
18M.3.sl.TZ2.6b:
Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon–carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid Mr = 304.5)
-
18M.3.sl.TZ2.6f:
Explain why lipids provide more energy than carbohydrates and proteins.
-
18M.3.sl.TZ2.6e:
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
-
18M.3.sl.TZ2.7a:
Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
-
18M.3.sl.TZ2.7c:
Outline why amino acids have high melting points.
-
18M.3.hl.TZ2.8e:
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
-
18M.3.hl.TZ2.8f:
UV-Vis spectroscopy can be used to determine the unknown concentration of a substance in a solution.
Calculate the concentration of an unknown sample of pepsin with an absorbance of 0.725 using section 1 of the data booklet.
Cell length = 1.00 cm
Molar absorptivity (extinction coefficient) of the sample = 49650 dm3 cm−1 mol−1
-
18M.3.hl.TZ2.8g:
A different series of pepsin samples is used to develop a calibration curve.
Estimate the concentration of an unknown sample of pepsin with an absorbance of 0.30 from the graph.
- 18N.3.sl.TZ0.6a: Describe the interaction responsible for the secondary structure of a protein.
- 18N.3.hl.TZ0.10c.i: Outline the difference between their structures.
- 18N.3.sl.TZ0.7c: Outline one effect of increased levels of low-density lipoproteins in the blood.
- 18N.3.hl.TZ0.10b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.hl.TZ0.7a: State the feature of DNA that determines the primary structure of proteins synthesised by a cell.
-
18N.3.hl.TZ0.11b:
Explain why carbon monoxide is very toxic and how it may be possible to treat carbon monoxide poisoning.
- 18N.3.hl.TZ0.8b: Explain the action of an enzyme and state one of its limitations.
- 18N.3.sl.TZ0.6b.ii: Enzymes are widely used in washing detergents. Outline how they improve the efficiency of the...
-
18N.3.sl.TZ0.7b.i:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
- 18N.3.hl.TZ0.11a: A graph showing saturation of oxygen against partial pressure of oxygen is shown. Explain the...
- 18N.3.sl.TZ0.8a: Name the type of link between the two monosaccharide residues.
- 18N.3.sl.TZ0.7b.ii: State two functions of lipids in the body.
- 18N.3.sl.TZ0.7a: A phospholipid generally consists of two hydrophobic fatty acids and a hydrophilic...
- 18N.3.sl.TZ0.8b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.hl.TZ0.10a: Name the type of link between the two monosaccharide residues.
- 18N.3.hl.TZ0.9c: Outline one effect of increased levels of low-density lipoproteins in the blood.
- 18N.3.sl.TZ0.5c: Explain how a xenobiotic is biomagnified.
- 18N.3.hl.TZ0.10c.ii: Outline why cellulose is an essential part of human diet.
- 18N.3.hl.TZ0.7b: Suggest one concern about the use of genetically modified, GM, food.
-
18N.3.sl.TZ0.5b:
Suggest why it is advisable for those living in northerly or southerly latitudes (that is away from the equator) to take vitamin D supplements during the winter.
- 18N.3.sl.TZ0.5a: The formation of proteins from amino acids is an example of an anabolic reaction in the human...
- 18N.3.sl.TZ0.6b.i: Explain the action of an enzyme and state one of its limitations.
- 18N.3.hl.TZ0.8a: Describe the interaction responsible for the secondary structure of a protein.
-
18N.3.hl.TZ0.8c:
Contrast the actions of non-competitive and competitive inhibitors of an enzyme and state their effects on the maximum rate of reaction, Vmax, and the Michaelis–Menten constant, Km.
-
18N.3.hl.TZ0.9a:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
- 18N.3.hl.TZ0.9b: State two functions of lipids in the body.
-
19M.3.hl.TZ1.9a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.hl.TZ1.9b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.hl.TZ1.12a(i):
A Michaelis–Menten plot for an enzyme-catalysed reaction is shown.
Sketch a curve to show the effect of a competitive inhibitor.
-
19M.3.hl.TZ1.8d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.hl.TZ1.9c:
Aspartic acid is obtained synthetically as a racemic mixture. Draw the three‑dimensional shape of each isomer showing their spatial relationship to each other. Use section 33 of the data booklet.
-
19M.3.hl.TZ1.10b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.hl.TZ1.14b:
Outline one benefit of the use of these products.
-
19M.3.hl.TZ1.8c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.hl.TZ1.11a:
The absorption spectrum of β-carotene is shown below.
Explain its colour in terms of its absorption bands. Use section 17 of the data booklet.
-
19M.3.hl.TZ1.11b:
The absorption spectrum of chlorophyll a is shown below.
Suggest how the combination of chlorophyll a and carotenoids is beneficial for photosynthesis.
-
19M.3.hl.TZ1.19b(ii):
Identify the splitting pattern of signals X and Y.
X:
Y:
-
19M.3.hl.TZ1.10a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.hl.TZ1.14a:
Outline what is meant by genetically modified organisms.
-
19M.3.hl.TZ1.8b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.hl.TZ1.10c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline one effect of trans-fatty acids on health.
-
19M.3.hl.TZ1.19b(i):
Deduce the protons responsible for signals X and Y by marking them on the structure of aspirin in (a). Use section 27 of the data booklet.
-
19M.3.hl.TZ1.12a(ii):
Suggest, based on the Michaelis–Menten plot, how a competitive inhibitor such as ethanol reduces the toxicity of methanol.
-
19M.3.hl.TZ1.12b:
Enzymatic activity is studied in buffered aqueous solutions.
Calculate the ratio in which 0.1 mol dm−3 NaH2PO4 (aq) and 0.1 mol dm−3 Na2HPO4 (aq) should be mixed to obtain a buffer with pH = 6.10. Use section 1 of the data booklet.
pKa (NaH2PO4) = 7.20
-
19M.3.hl.TZ1.8a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.hl.TZ2.10a(i):
Outline which pKa value should be used when calculating the pH of the solution, giving your reason.
-
19M.3.hl.TZ2.13a:
Outline why the complex formed between Fe2+ and oxygen is red. Refer to the diagram above and section 17 of the data booklet.
-
19M.3.hl.TZ1.13:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.hl.TZ2.9a(i):
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.hl.TZ2.11c:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
-
19M.3.hl.TZ2.11a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.hl.TZ2.11b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.hl.TZ2.9a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.hl.TZ2.12a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.hl.TZ2.12c:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19M.3.hl.TZ2.9c:
State and explain how a competitive inhibitor affects the maximum rate, Vmax, of an enzyme-catalyzed reaction.
-
19M.3.hl.TZ2.10b:
Describe what is meant by the genetic code and how it relates to protein synthesis.
-
19M.3.hl.TZ2.11a(ii):
Identify the type of reaction in (a).
-
19M.3.hl.TZ2.10a(ii):
Calculate the pH of the glutamine solution using section 1 of the data booklet.
- 19M.3.hl.TZ2.13b(ii): Sketch another line to show the effect of an increase in body temperature on the oxygen...
-
19M.3.hl.TZ2.13b(i):
Explain the shape of the curve.
-
19M.3.hl.TZ2.11d:
Phospholipids are also found in lipoprotein structures.
Describe one effect of increased levels of low-density lipoprotein (LDL) on health.
-
19M.3.hl.TZ2.9b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19M.3.hl.TZ2.9d(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
-
19M.3.hl.TZ2.9d(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.hl.TZ2.12b:
Classify, giving your reason, the hexose (six-membered) ring of sucrose as an α or β isomer.
-
19M.3.sl.TZ1.8a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.sl.TZ1.7b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.sl.TZ1.10:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.sl.TZ1.9a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.sl.TZ1.7c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.sl.TZ1.7d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.sl.TZ1.7a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.sl.TZ1.8b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.sl.TZ1.9c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline two effects of trans-fatty acids on health.
-
19M.3.sl.TZ1.9b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.sl.TZ2.7a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.sl.TZ2.6a(i) :
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.sl.TZ2.6a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.sl.TZ2.7b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.sl.TZ2.8a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.sl.TZ2.6c(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.sl.TZ2.7a(ii):
Identify the type of reaction in (a).
-
19M.3.sl.TZ2.7d:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
-
19M.3.sl.TZ2.8b:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19M.3.sl.TZ2.6c(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
-
19M.3.sl.TZ2.6b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19M.3.sl.TZ2.7e:
Phospholipids are also found in lipoprotein structures.
Describe two effects of increased levels of low-density lipoprotein (LDL) on health.
-
19M.3.sl.TZ2.7c:
Predict, giving a reason, the relative energy density of a carbohydrate and a lipid of similar molar mass.
-
19N.3.hl.TZ0.11b:
Compare the effects of competitive and non-competitive inhibitors.
- 19N.3.sl.TZ0.9c: Explain why stearic acid has a higher melting point than oleic acid.
- 19N.3.hl.TZ0.10b(ii): Suggest why alanine and glycine separate slightly at pH 6.5.
- 19N.3.sl.TZ0.8a: The graph shows the relationship between the temperature and the rate of an enzyme-catalysed...
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
- 19N.3.hl.TZ0.14a: The graph shows the change in oxygen partial pressure in blood, measured at different pH...
-
19N.3.sl.TZ0.7b:
The isoelectric point of amino acids is the intermediate pH at which an amino acid is electrically neutral.
Suggest why Asp and Phe have different isoelectric points.
- 19N.3.hl.TZ0.13b: Explain how the double-helical structure of DNA is stabilized once formed.
-
19N.3.sl.TZ0.7a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
- 19N.3.sl.TZ0.10b: Explain the biomagnification of the pesticide DDT.
-
19N.3.sl.TZ0.8b:
Explain why a change in pH affects the tertiary structure of an enzyme in solution.
-
19N.3.sl.TZ0.9a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
- 19N.3.sl.TZ0.9d(i): State one similarity and one difference in composition between phospholipids and...
-
19N.3.sl.TZ0.9d(ii):
Identify a reagent that hydrolyses triglycerides.
- 19N.3.sl.TZ0.8c: State one use of enzymes in reducing environmental problems.
- 19N.3.sl.TZ0.9b: State one impact on health of the increase in LDL cholesterol concentration in blood.
- 19N.3.sl.TZ0.10c: Explain why maltose, C12H22O11, is soluble in water.
- 19N.3.hl.TZ0.10b(i): Describe, using another method, how a mixture of four amino acids, alanine, arginine, glutamic...
- 19N.3.hl.TZ0.11a: Outline the significance of the Michaelis constant Km.
-
19N.3.hl.TZ0.12b:
The chemical change in stored fats causes rancidity characterized by an unpleasant smell or taste.
Compare hydrolytic and oxidative rancidity.
- 19N.3.hl.TZ0.15b: Compare and contrast the structures of starch and cellulose. One similarity: One difference:
-
19N.3.hl.TZ0.13a:
List two components of nucleotides.
- 19N.3.sl.TZ0.10a: State the name of one functional group common to all three vitamins shown in section 35 of the...
- 19N.3.hl.TZ0.15a: Describe the function of chlorophyll in photosynthesis.
-
19N.3.hl.TZ0.10a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
-
19N.3.hl.TZ0.12a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
- 19N.3.hl.TZ0.12c: State one similarity and one difference in composition between phospholipids and...
- 19N.3.hl.TZ0.14c: Vitamins are organic compounds essential in small amounts. State the name of one functional...
- 19N.3.hl.TZ0.14b: Explain the biomagnification of the pesticide DDT.
- 19N.3.hl.TZ0.15c: Explain why maltose, C12H22O11, is soluble in water.
Sub sections and their related questions
B.1 Introduction to biochemistry
- 16N.3.sl.TZ0.9a: State the raw materials and source of energy used in the process described above.
-
17M.3.sl.TZ1.12c.ii:
Biodegradable boxes made from polylactic acid, PLA, disintegrate when exposed to water.
State the formula of the product formed when water reacts with PLA.
-
17M.3.sl.TZ1.13a:
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
-
17M.3.sl.TZ2.9b:
Identify the type of reaction which occurs.
-
17N.3.sl.TZ0.9a:
Describe what is meant by a condensation reaction.
-
18M.3.sl.TZ1.6c.i:
State the name of the process used to break down the insulin protein into its constituent amino acids.
-
18M.3.sl.TZ2.6a:
Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
- 18N.3.sl.TZ0.5a: The formation of proteins from amino acids is an example of an anabolic reaction in the human...
-
19M.3.hl.TZ1.8b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.hl.TZ2.11a(ii):
Identify the type of reaction in (a).
-
19M.3.sl.TZ1.7b:
Formulate the equation for the complete hydrolysis of a starch molecule, (C6H10O5)n.
-
19M.3.sl.TZ2.7a(ii):
Identify the type of reaction in (a).
B.2 Proteins and enzymes
-
16N.3.sl.TZ0.10b:
A mixture of amino acids is separated by gel electrophoresis at pH 6.0. The amino acids are then stained with ninhydrin.
(i) On the diagram below draw the relative positions of the following amino acids at the end of the process: Val, Asp, Lys and Thr.
(ii) Suggest why glycine and isoleucine separate slightly at pH 6.5.
- 16N.3.sl.TZ0.10c: Determine the number of different tripeptides that can be made from twenty different amino acids.
-
16N.3.sl.TZ0.10d:
The fibrous protein keratin has a secondary structure with a helical arrangement.
(i) State the type of interaction responsible for holding the protein in this arrangement.
(ii) Identify the functional groups responsible for these interactions.
-
17M.3.sl.TZ1.13b.i:
Identify the name of the amino acid that does not move under the influence of the applied voltage.
-
17M.3.sl.TZ1.13b.ii:
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
-
17M.3.sl.TZ1.13c:
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
-
17M.3.sl.TZ2.7a:
Deduce the structural formula of the dipeptide Cys-Lys.
-
17M.3.sl.TZ2.7b:
Identify the type of bond between two cysteine residues in the tertiary structure of a protein.
-
17M.3.sl.TZ2.7c:
Deduce the structural formula of the predominant form of cysteine at pH 1.0.
-
17M.3.sl.TZ2.7d:
A mixture of the three amino acids, cysteine, glutamine and lysine, was placed in the centre of a square plate covered in polyacrylamide gel. The gel was saturated with a buffer solution of pH 6.0. Electrodes were connected to opposite sides of the gel and a potential difference was applied.
Sketch lines on the diagram to show the relative positions of the three amino acids after electrophoresis.

-
17M.3.hl.TZ2.8c.i:
An aqueous buffer solution contains both the zwitterion and the anionic forms of alanine. Draw the zwitterion of alanine.
- 17N.3.sl.TZ0.11: Enzyme activity depends on many factors. Explain how pH change causes loss of activity of an enzyme.
-
18M.3.hl.TZ2.8c:
Draw the structures of the main form of glycine in buffer solutions of pH 1.0 and 6.0.
The pKa of glycine is 2.34.
-
18M.3.sl.TZ1.6a:
Draw the structural formula of a dipeptide containing the residues of valine, Val, and asparagine, Asn, using section 33 of the data booklet.
-
18M.3.sl.TZ1.6b:
Deduce the strongest intermolecular forces that would occur between the following amino acid residues in a protein chain.
-
18M.3.sl.TZ1.6c.ii:
Outline how the amino acids may be identified from a paper chromatogram.
-
18M.3.sl.TZ2.7a:
Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
-
18M.3.sl.TZ2.7c:
Outline why amino acids have high melting points.
- 18N.3.sl.TZ0.6a: Describe the interaction responsible for the secondary structure of a protein.
- 18N.3.sl.TZ0.6b.i: Explain the action of an enzyme and state one of its limitations.
- 18N.3.hl.TZ0.8a: Describe the interaction responsible for the secondary structure of a protein.
- 18N.3.hl.TZ0.8b: Explain the action of an enzyme and state one of its limitations.
-
19M.3.hl.TZ1.9a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.hl.TZ1.9b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.hl.TZ2.9a(i):
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.hl.TZ2.9a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.hl.TZ2.9b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19M.3.sl.TZ1.8a:
Draw a circle around the functional group formed between the amino acids and state its name.
Name:
-
19M.3.sl.TZ1.8b:
A mixture of phenylalanine and aspartic acid is separated by gel electrophoresis with a buffer of pH = 5.5.
Deduce their relative positions after electrophoresis, annotating them on the diagram. Use section 33 of the data booklet.
-
19M.3.sl.TZ2.6a(i) :
Some proteins form an α-helix. State the name of another secondary protein structure.
-
19M.3.sl.TZ2.6a(ii):
Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
-
19M.3.sl.TZ2.6b:
Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
-
19N.3.sl.TZ0.7a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
- 19N.3.hl.TZ0.10b(i): Describe, using another method, how a mixture of four amino acids, alanine, arginine, glutamic...
- 19N.3.hl.TZ0.10b(ii): Suggest why alanine and glycine separate slightly at pH 6.5.
-
19N.3.sl.TZ0.7b:
The isoelectric point of amino acids is the intermediate pH at which an amino acid is electrically neutral.
Suggest why Asp and Phe have different isoelectric points.
- 19N.3.sl.TZ0.8a: The graph shows the relationship between the temperature and the rate of an enzyme-catalysed...
-
19N.3.sl.TZ0.8b:
Explain why a change in pH affects the tertiary structure of an enzyme in solution.
-
19N.3.hl.TZ0.10a:
Draw the structure of the dipeptide Asp–Phe using section 33 of the data booklet.
-
20N.3.sl.TZ0.5a(i):
Proteins are polymers of amino acids. A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
-
20N.3.sl.TZ0.5a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.sl.TZ0.5b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
-
20N.3.sl.TZ0.5c:
Proteins are polymers of amino acids.
Describe how the tertiary structure differs from the quaternary structure in hemoglobin.
-
20N.3.hl.TZ0.6a(i):
Proteins are polymers of amino acids.
A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
-
20N.3.hl.TZ0.6a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.hl.TZ0.6b:
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
B.3 Lipids
- 16N.3.sl.TZ0.8a: Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed...
-
16N.3.sl.TZ0.8b:
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
-
17M.3.sl.TZ1.11a:
List the building blocks of triglycerides and carbohydrates.
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ1.11b.ii:
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
-
17M.3.sl.TZ1.11c:
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16. -
17M.3.sl.TZ2.8a:
Explain which one of these fatty acids has the highest boiling point.
-
17M.3.sl.TZ2.8b:
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 moldm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
-
17M.3.sl.TZ2.9a:
Glycerol is one product of the reaction. Identify the two other organic products.
-
17M.3.sl.TZ2.9b:
Identify the type of reaction which occurs.
- 17N.3.sl.TZ0.8a.ii: The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per...
- 17N.3.sl.TZ0.8b.i: State the type of reaction occurring during the titration.
-
17N.3.sl.TZ0.8b.ii:
Calculate the volume of iodine solution used to reach the end-point.
-
17N.3.sl.TZ0.8c:
Outline the importance of linoleic acid for human health.
-
18M.3.sl.TZ1.7a.i:
Identify the type of rancidity occurring in saturated lipids and the structural feature that causes it.
-
18M.3.sl.TZ1.7a.ii:
State one factor that increases the rate at which saturated lipids become rancid.
-
18M.3.sl.TZ1.7b:
Butter contains varying proportions of oleic, myristic, palmitic and stearic acids. Explain in terms of their structures why stearic acid has a higher melting point than oleic acid, using section 34 of the data booklet.
-
18M.3.sl.TZ1.7c.i:
Fish oil is an excellent dietary source of omega-3 fatty acids. Outline one impact on health of consuming omega-3 fatty acids.
-
18M.3.sl.TZ2.6b:
Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon–carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid Mr = 304.5)
-
18M.3.sl.TZ2.6c:
Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
-
18M.3.sl.TZ2.6d:
Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
-
18M.3.sl.TZ2.6f:
Explain why lipids provide more energy than carbohydrates and proteins.
- 18N.3.sl.TZ0.7a: A phospholipid generally consists of two hydrophobic fatty acids and a hydrophilic...
-
18N.3.sl.TZ0.7b.i:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
- 18N.3.sl.TZ0.7b.ii: State two functions of lipids in the body.
- 18N.3.sl.TZ0.7c: Outline one effect of increased levels of low-density lipoproteins in the blood.
- 18N.3.hl.TZ0.9b: State two functions of lipids in the body.
- 18N.3.hl.TZ0.9c: Outline one effect of increased levels of low-density lipoproteins in the blood.
-
18N.3.hl.TZ0.9a:
The iodine number is the maximum mass of iodine that reacts with 100 g of an unsaturated compound.
Determine the iodine number of stearidonic acid, C17H27COOH.
-
19M.3.hl.TZ1.10a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.hl.TZ1.10b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.hl.TZ1.10c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline one effect of trans-fatty acids on health.
-
19M.3.hl.TZ2.11a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.hl.TZ2.11b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.hl.TZ2.11d:
Phospholipids are also found in lipoprotein structures.
Describe one effect of increased levels of low-density lipoprotein (LDL) on health.
-
19M.3.sl.TZ1.9a:
The melting points of cocoa butter and coconut oil are 34 °C and 25 °C respectively.
Explain this in terms of their saturated fatty acid composition.
-
19M.3.sl.TZ1.9b:
Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
-
19M.3.sl.TZ1.9c:
The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline two effects of trans-fatty acids on health.
-
19M.3.sl.TZ2.7a(i):
Deduce the structural formula of phosphatidylcholine.
-
19M.3.sl.TZ2.7b:
Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
-
19M.3.sl.TZ2.7c:
Predict, giving a reason, the relative energy density of a carbohydrate and a lipid of similar molar mass.
-
19M.3.sl.TZ2.7e:
Phospholipids are also found in lipoprotein structures.
Describe two effects of increased levels of low-density lipoprotein (LDL) on health.
-
19N.3.sl.TZ0.9a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
-
19N.3.hl.TZ0.12b:
The chemical change in stored fats causes rancidity characterized by an unpleasant smell or taste.
Compare hydrolytic and oxidative rancidity.
- 19N.3.sl.TZ0.9d(i): State one similarity and one difference in composition between phospholipids and...
- 19N.3.sl.TZ0.9b: State one impact on health of the increase in LDL cholesterol concentration in blood.
- 19N.3.sl.TZ0.9c: Explain why stearic acid has a higher melting point than oleic acid.
-
19N.3.sl.TZ0.9d(ii):
Identify a reagent that hydrolyses triglycerides.
-
19N.3.hl.TZ0.12a:
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
- 19N.3.hl.TZ0.12c: State one similarity and one difference in composition between phospholipids and...
-
20N.3.sl.TZ0.6a:
Deduce the products of the hydrolysis of a non-substituted phospholipid, where and represent long alkyl chains.
-
20N.3.sl.TZ0.6b(i):
A representation of a phospholipid bilayer cell membrane is shown:
© International Baccalaureate Organization 2020.
Identify the components of the phospholipid labelled A and B.
-
20N.3.sl.TZ0.6c:
Phospholipids help maintain cellular environments while fatty acid lipids have important roles in energy storage and electrical insulation. Discuss the structural properties of saturated fats needed for these roles.
B.4 Carbohydrates
-
16N.3.sl.TZ0.9b:
The structures of two molecules, X and Y, are shown below.
(i) Justify why both these molecules are carbohydrates.
(ii) Distinguish between these molecules in terms of their functional groups.
-
16N.3.sl.TZ0.9c:
Amylose is an unbranched polysaccharide composed of repeating units of glucose.
(i) Draw the structure of the repeating unit of amylose. Use section 34 of the data booklet.
(ii) Amylose is a major component of starch. Corn starch can be used to make replacements for plastics derived from oil, especially for packaging. Discuss one potential advantage and one disadvantage of this use of starch.
-
17M.3.sl.TZ1.11a:
List the building blocks of triglycerides and carbohydrates.
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ1.12a.i:
Deduce the straight chain structure of ribose from its ring structure drawn in section 34 of the data booklet.
-
17M.3.sl.TZ1.12a.ii:
Using the partial structure given, complete the structural formula of the molecule formed from the condensation of two cyclic -glucose molecules.

-
17M.3.sl.TZ1.12b:
Constructing models that allow visualizations of the stereochemistry of carbohydrates is essential to understand their structural roles in cells.
Describe how Haworth projections help focus on the position of attached groups.
-
17M.3.sl.TZ2.10a:
Identify the functional groups which are present in only one structure of glucose.
-
17M.3.sl.TZ2.10b:
Sucrose is a disaccharide formed from -glucose and β-fructose.
Deduce the structural formula of sucrose.
- 17N.3.sl.TZ0.9b: Draw the structure of galactose on the skeleton provided.
-
18M.3.sl.TZ1.8a:
State the specific type of linkage formed between α-glucose fragments in both maltose and amylose.
-
18M.3.sl.TZ1.8b:
A person with diabetes suffering very low blood sugar (hypoglycaemia) may be advised to consume glucose immediately and then eat a small amount of starchy food such as a sandwich. Explain this advice in terms of the properties of glucose and starch.
-
18M.3.sl.TZ2.6e:
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
- 18N.3.sl.TZ0.8a: Name the type of link between the two monosaccharide residues.
- 18N.3.sl.TZ0.8b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.hl.TZ0.10a: Name the type of link between the two monosaccharide residues.
- 18N.3.hl.TZ0.10b: Outline how the two monomer structures, galactose and glucose, differ.
-
19M.3.hl.TZ1.8a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.hl.TZ1.8c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.hl.TZ2.12a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.hl.TZ2.12c:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19M.3.sl.TZ1.7a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.sl.TZ1.7c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.sl.TZ2.8a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.sl.TZ2.8b:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
- 19N.3.sl.TZ0.10c: Explain why maltose, C12H22O11, is soluble in water.
- 19N.3.hl.TZ0.15c: Explain why maltose, C12H22O11, is soluble in water.
-
20N.3.sl.TZ0.7b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
-
20N.3.sl.TZ0.7b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Sucrose is a disaccharide formed in the reaction of glucose with fructose.
Identify the reaction type and the newly formed functional group that joins the monosaccharide units in the product.
-
20N.3.hl.TZ0.8b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
B.5 Vitamins
-
17M.3.sl.TZ1.13d:
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
-
17M.3.sl.TZ2.11:
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
-
17N.3.sl.TZ0.10a:
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
- 17N.3.sl.TZ0.10b: State one function of vitamin D in the body.
-
18M.3.sl.TZ1.7c.ii:
Predict the solubility of retinol (vitamin A) in body fat, giving a reason. Use section 35 of the data booklet.
-
18M.3.sl.TZ2.9:
Explain the solubility of vitamins A and C using section 35 of the data booklet.
-
18N.3.sl.TZ0.5b:
Suggest why it is advisable for those living in northerly or southerly latitudes (that is away from the equator) to take vitamin D supplements during the winter.
-
19M.3.hl.TZ1.13:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.hl.TZ2.11c:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
-
19M.3.sl.TZ1.10:
Ascorbic acid and retinol are two important vitamins.
Explain why ascorbic acid is soluble in water and retinol is not. Use section 35 of the data booklet.
-
19M.3.sl.TZ2.7d:
Lecithin aids the body’s absorption of vitamin E.
Suggest why vitamin E is fat-soluble.
- 19N.3.sl.TZ0.10a: State the name of one functional group common to all three vitamins shown in section 35 of the...
- 19N.3.hl.TZ0.14c: Vitamins are organic compounds essential in small amounts. State the name of one functional...
- 20N.3.sl.TZ0.7a: The diverse functions of biological molecules depend on their structure and shape. Classify...
- 20N.3.hl.TZ0.8a: The diverse functions of biological molecules depend on their structure and shape. Classify...
B.6 Biochemistry and the environment
-
16N.3.sl.TZ0.9c:
Amylose is an unbranched polysaccharide composed of repeating units of glucose.
(i) Draw the structure of the repeating unit of amylose. Use section 34 of the data booklet.
(ii) Amylose is a major component of starch. Corn starch can be used to make replacements for plastics derived from oil, especially for packaging. Discuss one potential advantage and one disadvantage of this use of starch.
-
17M.3.sl.TZ1.12c.i:
State one advantage of starch based polymers besides being biodegradable.
-
17M.3.sl.TZ1.13e:
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
-
17M.3.sl.TZ1.13f:
Outline how lead ions could be removed from an individual suffering from lead poisoning.
-
17M.3.sl.TZ2.10c:
Starch is a constituent of many plastics. Suggest one reason for including starch in plastics.
-
17M.3.sl.TZ2.10d:
Suggest one of the challenges scientists face when scaling up the synthesis of a new compound.
-
17N.3.sl.TZ0.9c:
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
-
18M.3.sl.TZ1.7c.iii:
Explain why sharks and swordfish sometimes contain high concentrations of mercury and polychlorinated biphenyls (PCBs).
-
18M.3.sl.TZ1.7c.iv:
Plastics are another source of marine pollution. Outline one way in which plastics can be made more biodegradable.
-
18M.3.sl.TZ2.8:
Green Chemistry reduces the production of hazardous materials and chemical waste.
Outline two specific examples or technological processes of how Green Chemistry has accomplished this environmental impact.
- 18N.3.sl.TZ0.5c: Explain how a xenobiotic is biomagnified.
- 18N.3.sl.TZ0.6b.ii: Enzymes are widely used in washing detergents. Outline how they improve the efficiency of the...
-
19M.3.hl.TZ1.8d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.hl.TZ2.9d(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.hl.TZ2.9d(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
-
19M.3.sl.TZ1.7d:
Explain how the inclusion of starch in plastics makes them biodegradable.
-
19M.3.sl.TZ2.6c(i):
Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
-
19M.3.sl.TZ2.6c(ii):
Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
- 19N.3.sl.TZ0.10b: Explain the biomagnification of the pesticide DDT.
- 19N.3.sl.TZ0.8c: State one use of enzymes in reducing environmental problems.
- 19N.3.hl.TZ0.14b: Explain the biomagnification of the pesticide DDT.
-
20N.3.sl.TZ0.8a:
Calculate the BMF if a shark consumes mackerel in one year. Each mackerel weighs on average. The per body weight. Assume chemical remains in the shark’s body for two years.
- 20N.3.sl.TZ0.8b: Suggest, with a reason, if fat-soluble or water-soluble xenobiotics would have a larger BMF.
B.7 Proteins and enzymes (HL only)
-
16N.3.hl.TZ0.13c:
Amino acids act as buffers in solution. In aspartic acid, the side chain (R group) carboxyl has pKa = 4.0. Determine the percentage of the side chain carboxyl that will be ionized (–COO–) in a solution of aspartic acid with pH = 3.0. Use section 1 of the data booklet.
-
16N.3.hl.TZ0.14a:
(i) Estimate the Km values of the two enzymes.
(ii) Suggest, with a reason, which enzyme will be more responsive to changes in the concentration of glucose in the blood.
-
16N.3.hl.TZ0.14b:
(i) Outline what is meant by product inhibition as it applies to hexokinase.
(ii) Product inhibition of hexokinase does not affect its Km value. Using this information, deduce the type of binding site that the inhibitor attaches to.
-
17M.3.hl.TZ1.15a:
Deduce the pH range in which glycine is an effective buffer in basic solution.
-
17M.3.hl.TZ1.15b:
Enzymes are biological catalysts.
The data shows the effect of substrate concentration, [S], on the rate, v, of an enzyme-catalysed reaction.
Determine the value of the Michaelis constant (Km) from the data. A graph is not required.
-
17M.3.hl.TZ1.15c:
Outline the action of a non-competitive inhibitor on the enzyme-catalysed reaction.
-
17M.3.hl.TZ2.8c.ii:
Calculate the pH of a buffer solution which contains 0.700 mol dm–3 of the zwitterion and 0.500 mol dm–3 of the anionic form of alanine.
Alanine pKa = 9.87.
-
17M.3.hl.TZ2.13a:
Determine the value of the Michaelis constant, Km, including units, from the graph.
-
17M.3.hl.TZ2.13b:
Sketch a second graph on the same axes to show how the reaction rate varies when a competitive inhibitor is present.
-
17M.3.hl.TZ2.13c:
Outline the significance of the value of Km.
-
17N.3.hl.TZ0.11a:
Determine the value of the Michaelis constant, Km, by annotating the graph.
-
17N.3.hl.TZ0.11b.i:
The malonate ion acts as an inhibitor for the enzyme.
Suggest, on the molecular level, how the malonate ion is able to inhibit the enzyme.
-
17N.3.hl.TZ0.11b.ii:
Draw a curve on the graph above showing the effect of the presence of the malonate ion inhibitor on the rate of reaction.
-
18M.3.hl.TZ1.9a:
Explain with reference to the binding site on the enzyme how a non-competitive inhibitor lowers the value of Vmax.
-
18M.3.hl.TZ1.9b:
Outline the significance of the value of the Michaelis constant, Km.
-
18M.3.hl.TZ2.8d:
Calculate the pH of a buffer system with a concentration of 1.25 × 10−3 mol dm−3 carbonic acid and 2.50 × 10−2 mol dm−3 sodium hydrogen carbonate. Use section 1 of the data booklet.
pKa (carbonic acid) = 6.36
-
18M.3.hl.TZ2.8f:
UV-Vis spectroscopy can be used to determine the unknown concentration of a substance in a solution.
Calculate the concentration of an unknown sample of pepsin with an absorbance of 0.725 using section 1 of the data booklet.
Cell length = 1.00 cm
Molar absorptivity (extinction coefficient) of the sample = 49650 dm3 cm−1 mol−1
-
18M.3.hl.TZ2.8g:
A different series of pepsin samples is used to develop a calibration curve.
Estimate the concentration of an unknown sample of pepsin with an absorbance of 0.30 from the graph.
-
18N.3.hl.TZ0.8c:
Contrast the actions of non-competitive and competitive inhibitors of an enzyme and state their effects on the maximum rate of reaction, Vmax, and the Michaelis–Menten constant, Km.
-
19M.3.hl.TZ1.12a(i):
A Michaelis–Menten plot for an enzyme-catalysed reaction is shown.
Sketch a curve to show the effect of a competitive inhibitor.
-
19M.3.hl.TZ1.12a(ii):
Suggest, based on the Michaelis–Menten plot, how a competitive inhibitor such as ethanol reduces the toxicity of methanol.
-
19M.3.hl.TZ1.12b:
Enzymatic activity is studied in buffered aqueous solutions.
Calculate the ratio in which 0.1 mol dm−3 NaH2PO4 (aq) and 0.1 mol dm−3 Na2HPO4 (aq) should be mixed to obtain a buffer with pH = 6.10. Use section 1 of the data booklet.
pKa (NaH2PO4) = 7.20
-
19M.3.hl.TZ2.9c:
State and explain how a competitive inhibitor affects the maximum rate, Vmax, of an enzyme-catalyzed reaction.
-
19M.3.hl.TZ2.10a(i):
Outline which pKa value should be used when calculating the pH of the solution, giving your reason.
-
19M.3.hl.TZ2.10a(ii):
Calculate the pH of the glutamine solution using section 1 of the data booklet.
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
- 19N.3.hl.TZ0.11a: Outline the significance of the Michaelis constant Km.
-
19N.3.hl.TZ0.11b:
Compare the effects of competitive and non-competitive inhibitors.
- 20N.3.hl.TZ0.10a: Identify the type of inhibition shown in the graph.
-
20N.3.hl.TZ0.10b(i):
Determine the value of and in the absence and presence of the inhibitor.
-
20N.3.hl.TZ0.10b(ii):
Outline the significance of the value of the Michaelis constant, .
B.8 Nucleic acids (HL only)
- 16N.3.hl.TZ0.15a: State the name of the component of DNA responsible for the migration of its fragments to the...
-
16N.3.hl.TZ0.15b:
In 2010, scientists claimed that they had discovered a species of bacteria capable of incorporating arsenic in place of phosphorus into the bacterial DNA. This claim has since proved controversial. Suggest one technique or evidence that might help support the claim.
-
17M.3.hl.TZ1.15d:
The sequence of nitrogenous bases in DNA determines hereditary characteristics.
Calculate the mole percentages of cytosine, guanine and thymine in a double helical DNA structure if it contains 17% adenine by mole.
-
17M.3.hl.TZ2.15a:
Outline how its structure allows it to be negatively charged in the body.
-
17M.3.hl.TZ2.15b:
Deduce the nucleotide sequence of a complementary strand of a fragment of DNA with the nucleotide sequence –GACGGATCA–.
-
17N.3.hl.TZ0.13:
The stability of DNA is due to interactions of its hydrophilic and hydrophobic components.
Outline the interactions of the phosphate groups in DNA with water and with surrounding proteins (histones).
-
18M.3.hl.TZ1.6d:
Describe how DNA determines the primary structure of a protein such as insulin.
-
18M.3.hl.TZ2.12:
DNA is a biopolymer made up of nucleotides. List two components of a nucleotide.
- 18N.3.hl.TZ0.7a: State the feature of DNA that determines the primary structure of proteins synthesised by a cell.
- 18N.3.hl.TZ0.7b: Suggest one concern about the use of genetically modified, GM, food.
-
19M.3.hl.TZ1.14a:
Outline what is meant by genetically modified organisms.
-
19M.3.hl.TZ1.14b:
Outline one benefit of the use of these products.
-
19M.3.hl.TZ2.10b:
Describe what is meant by the genetic code and how it relates to protein synthesis.
-
19N.3.hl.TZ0.13a:
List two components of nucleotides.
- 19N.3.hl.TZ0.13b: Explain how the double-helical structure of DNA is stabilized once formed.
-
20N.3.hl.TZ0.8b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Draw the nitrogenous base that is paired with guanine in DNA, showing the hydrogen bonds between the bases. Use section 34 of the data booklet.
B.9 Biological pigments (HL only)
- 16N.3.hl.TZ0.16a: Outline why this molecule absorbs visible light.
- 16N.3.hl.TZ0.16b: With reference to its chemical structure, outline whether this pigment is found in aqueous...
-
16N.3.hl.TZ0.16c:
A student investigated the ability of anthocyanins to act as pH indicators. He extracted juice from blackberries and used a UV-vis spectrophotometer to produce absorption spectra at different pH values. His results are shown below.
Deduce the colour of the juice at each pH, giving your reasoning. Use section 17 of the data booklet.
-
17M.3.hl.TZ1.16a:
Explain the shape of the curve at low oxygen partial pressure up to about 5 kPa.
-
17M.3.hl.TZ1.16b.i:
Sketch a graph on the axes above to show the effect of decreasing pH on the binding of oxygen to hemoglobin (the Bohr Effect).
-
17M.3.hl.TZ1.16b.ii:
Outline the effect of decreasing pH on the oxygen saturation of hemoglobin.
-
17M.3.hl.TZ2.14a:
Explain the shape of the curve from 0 to X kPa.
-
17M.3.hl.TZ2.14b:
Explain why carbon monoxide is toxic to humans.
-
17N.3.hl.TZ0.14a:
State the half-equation for the reduction of molecular oxygen to water in acidic conditions.
- 17N.3.hl.TZ0.14b: Outline the change in oxidation state of the iron ions in heme groups that occurs when molecular...
-
18M.3.hl.TZ1.10a:
Outline why anthocyanins are coloured.
-
18M.3.hl.TZ1.10b:
Explain why the blue colour of a quinoidal base changes to the red colour of a flavylium cation as pH decreases.
-
18M.3.hl.TZ2.11a:
Hemoglobin’s oxygen dissociation curve is shown at a given temperature. Sketch the curve on the graph at a higher temperature.

-
18M.3.hl.TZ2.11b:
Outline two differences between normal hemoglobin and foetal hemoglobin.
- 18N.3.hl.TZ0.11a: A graph showing saturation of oxygen against partial pressure of oxygen is shown. Explain the...
-
18N.3.hl.TZ0.11b:
Explain why carbon monoxide is very toxic and how it may be possible to treat carbon monoxide poisoning.
-
19M.3.hl.TZ1.11a:
The absorption spectrum of β-carotene is shown below.
Explain its colour in terms of its absorption bands. Use section 17 of the data booklet.
-
19M.3.hl.TZ1.11b:
The absorption spectrum of chlorophyll a is shown below.
Suggest how the combination of chlorophyll a and carotenoids is beneficial for photosynthesis.
-
19M.3.hl.TZ1.19b(i):
Deduce the protons responsible for signals X and Y by marking them on the structure of aspirin in (a). Use section 27 of the data booklet.
-
19M.3.hl.TZ1.19b(ii):
Identify the splitting pattern of signals X and Y.
X:
Y:
-
19M.3.hl.TZ2.13a:
Outline why the complex formed between Fe2+ and oxygen is red. Refer to the diagram above and section 17 of the data booklet.
-
19M.3.hl.TZ2.13b(i):
Explain the shape of the curve.
- 19M.3.hl.TZ2.13b(ii): Sketch another line to show the effect of an increase in body temperature on the oxygen...
- 19N.3.hl.TZ0.14a: The graph shows the change in oxygen partial pressure in blood, measured at different pH...
- 19N.3.hl.TZ0.15a: Describe the function of chlorophyll in photosynthesis.
- 20N.3.hl.TZ0.6c(i): Proteins are polymers of amino acids. Sketch and label two oxygen dissociation curves, one for...
-
20N.3.hl.TZ0.6c(ii):
Proteins are polymers of amino acids.
Explain why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin.
B.10 Stereochemistry in biomolecules (HL only)
-
17M.3.hl.TZ1.17:
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
-
17M.3.hl.TZ2.12a:
Identify the structural feature which enables rhodopsin to absorb visible light.
-
17M.3.hl.TZ2.12b:
Outline the change that occurs in the retinal residue during the absorption of visible light.
-
17N.3.hl.TZ0.15b:
Retinal is the key molecule involved in vision. Explain the roles of cis and trans-retinal in vision and how the isomers are formed in the visual cycle.
-
18M.3.hl.TZ1.8b:
Outline why cellulose fibres are strong.
-
18M.3.hl.TZ2.10b:
Explain how the structure of vitamin A is important to vision using section 35 of the data booklet.
-
18M.3.hl.TZ2.8e:
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
- 18N.3.hl.TZ0.10c.i: Outline the difference between their structures.
- 18N.3.hl.TZ0.10c.ii: Outline why cellulose is an essential part of human diet.
-
19M.3.hl.TZ1.9c:
Aspartic acid is obtained synthetically as a racemic mixture. Draw the three‑dimensional shape of each isomer showing their spatial relationship to each other. Use section 33 of the data booklet.
-
19M.3.hl.TZ2.12b:
Classify, giving your reason, the hexose (six-membered) ring of sucrose as an α or β isomer.
- 19N.3.hl.TZ0.15b: Compare and contrast the structures of starch and cellulose. One similarity: One difference:
- 20N.3.hl.TZ0.8c: The diverse functions of biological molecules depend on their structure and shape. Retinal is...
