| Date | May 2017 | Marks available | 2 | Reference code | 17M.3.sl.TZ1.15 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | State | Question number | 15 | Adapted from | N/A |
Question
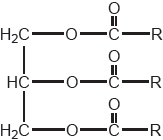
Vegetable oils, such as that shown, require conversion to biodiesel for use in current internal combustion engines.
State two reagents required to convert vegetable oil to biodiesel.
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
Determine the specific energy, in kJg−1, and energy density, in kJcm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 gcm−3; Molar mass = 299 gmol−1;
Enthalpy of combustion = 12.0 MJmol−1.
Markscheme
methanol
OR
ethanol
strong acid
OR
strong base
Accept “alcohol”.
Accept any specific strong acid or strong base other than HNO3/nitric acid.
[3 marks]
CH3(CH2)16COOCH3 / CH3OCO(CH2)16CH3
OR
CH3(CH2)16COOC2H5 / C2H5OCO(CH2)16CH3
Product must correspond to alcohol chosen in (a), but award mark for either structure if neither given for (a).
[1 mark]
lower viscosity
weaker intermolecular/dispersion/London/van der Waals’ forces
OR
smaller/shorter molecules
Accept “lower molecular mass/Mr” or “lower number of electrons”.
Accept converse arguments.
[2 marks]
Specific energy: «» = 40.1 «kJ g−1»
Energy density: «= 40.1 kJg−1 x 0.850 gcm−3» = 34.1 «kJcm−3»
Award [1] if both are in terms of a unit other than kJ (such as J or MJ).
[2 marks]