| Date | November 2020 | Marks available | 1 | Reference code | 20N.3.hl.TZ0.15 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Comment | Question number | 15 | Adapted from | N/A |
Question
Aspirin is formed by reacting salicylic acid with ethanoic anhydride. The structure of aspirin is given in section 37 of the data booklet.
Deduce the structural formula of the by-product of this reaction.
Aspirin crystals are rinsed with water after recrystallization to remove impurities.
Suggest why cold water is used.
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
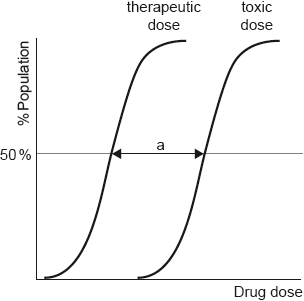
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
Explain how IR spectroscopy can be used to distinguish aspirin from salicylic acid.
Markscheme
OR
✔
Accept full OR condensed structural formula.
to avoid dissolving the crystals/aspirin ✔
Accept “to avoid loss of product” OR “aspirin is less soluble in cold water”.
✔
Accept a positive metal ion next to the such as “”.
Accept “” without showing the charges.
Accept notations such as “” OR “” OR “” but not “” OR “” OR “”.
low/medium risk «of overdosing» AND «estimated» lethal dose is times/much larger than therapeutic dose OR
times the dose results in chance of dying ✔
Accept “ and low/medium risk due to large therapeutic index”.
Do not accept “low/medium risk AND large therapeutic window”.
Do not accept “ times the dose” alone for the mark.
salicylic acid contains absorption in the range ✔
due to phenol/hydroxyl/ group not present in aspirin ✔
Award [2] for “additional «stretch» in IR for salicylic acid at higher wavenumber than corresponding «stretch» in aspirin” OR “aspirin has two absorption bands/one stronger absorption band in while salicylic acid has one/weaker absorption band in that region”.
Award [1 max] for “fingerprint regions will be different for both”.
Examiners report
Most candidates were able to deduce a correct structural formula (either full or condensed) for ethanoic acid. A minority did not read the question fully and gave the structure of aspirin instead of the by-product of the reaction. Another incorrect answer cited as the by-product was water.
Many were unable to explain why aspirin should be washed with cold water, namely, to avoid dissolving crystals. Surprisingly, the incorrect term "melt" was frequently used instead of "dissolve".
A drawing of the structure of the ionic form of aspirin was required for this question. This question was poorly answered by a significant number of candidates, and lots of basic chemical errors were seen, such as incorrect valencies, writing RCO- instead of RCOO-, showing a cationic structure instead of an anionic structure etc. A couple of candidates also lost the mark by drawing square brackets with a negative charge both inside and outside the bracket.
Few scored this mark. Most knew the overdose risk was low but referred to a large therapeutic window instead of a large therapeutic index. Many also did not quantify the therapeutic index by working out that the estimated lethal dose is actually 30 times the therapeutic dose.
This question which asked for an explanation of how IR spectroscopy can be used to distinguish aspirin from salicyclic acid was generally very well answered. The majority stated that salicyclic acid contains an absorption in the IR spectrum in the 3200-3600 cm-1 range due to the phenolic OH group, which is not present in aspirin. A few stated that aspirin has a methyl group and hence the CH stretch will appear in the 2850-3090 cm-1 region of the IR spectrum in aspirin (using Section 26 of the Data Booklet) which will not appear in the corresponding IR spectrum for salicyclic acid. This is somewhat incorrect as in salicyclic acid the benzene ring will also have CH bonds and the CH stretch for the benzene ring will occur in a similar region of the IR spectrum (as indicated in Section 26 of the Data Booklet) and hence cannot be used to distinguish fully between the two structures per se if using the Data Booklet range. Of course, in practice the alkyl CH stretch would be at a slightly lower wavenumber (e.g. 2850-2950 cm-1) in the IR spectrum compared to the aromatic CH stretch (3030 cm-1), but virtually no candidate gave this type of precise detail.