| Date | May 2017 | Marks available | 1 | Reference code | 17M.3.sl.TZ1.13 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Comment | Question number | 13 | Adapted from | N/A |
Question
Peptidase enzyme in the digestive system hydrolyses peptide bonds.
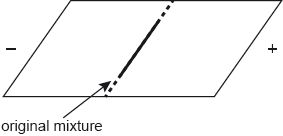
A tripeptide Ala-Asp-Lys was hydrolysed and electrophoresis of the mixture of the amino acids was carried out at a pH of 6.0. Refer to section 33 of the data booklet.
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
Identify the name of the amino acid that does not move under the influence of the applied voltage.
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
Outline how lead ions could be removed from an individual suffering from lead poisoning.
Markscheme
catabolism/catabolic
[1 mark]
alanine
Do not accept ala.
[1 mark]
Lys/lysine
pH «buffer» < pI «Lys»
OR
buffer more acidic than Lys «at isoelectric point»
OR
«Lys» exists as
OR
«Lys» charged positively/has +1/1+ «overall» charge «and moves to negative electrode»
Do not apply ECF from M1.
Accept converse argument.
Do not accept just “has H3N+ group” for M2 (as H3N+ is also present in zwitterion).
Do not penalize if COOH is given in the structure of lysine at pH 6 instead of COO–.
[2 marks]
highest frequency of successful collisions between active site and substrate
OR
highest frequency of collisions between active site and substrate with sufficient energy/ AND correct orientation/conformation
OR
optimal shape/conformation of the active site «that matches the substrate»
OR
best ability of the active site to bind «to the substrate»
Accept “number of collisions per unit time” for “frequency”.
Do not accept “all active sites are occupied”.
[1 mark]
ascorbic acid/vitamin C
[1 mark]
react/bind/chelate with enzyme
OR
disrupt ionic salt bridges
OR
affect shape of tertiary/quaternary structures
OR
precipitate enzymes
OR
break/disrupt disulfide bridges/bonds
Do not accept “changes shape of active site” by itself.
[1 mark]
«use of» host-guest chemistry
OR
chelation «therapy»
Accept specific medication/chelating agent such as EDTA, CaNa2 EDTA, succimer, D-penicillamine, dimercaprol.
[1 mark]