| Date | May 2017 | Marks available | 1 | Reference code | 17M.3.hl.TZ2.17 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Outline | Question number | 17 | Adapted from | N/A |
Question
There are many sources of energy available.
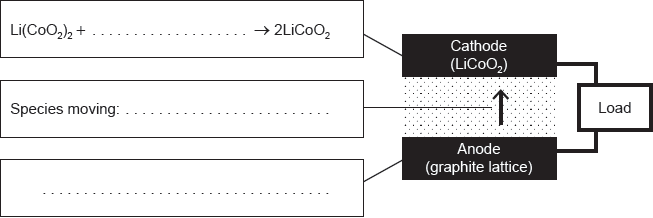
Methanol fuel cells provide a portable energy source. The process can be represented by the overall equation CH3OH(aq) + O2(g) → CO2(g) + 2H2O(g).
Deduce the half-cell equations occurring at each electrode during discharge.
Outline the function of the proton-exchange membrane (PEM) in the fuel cell.
Explain how the flow of ions allows for the operation of the fuel cell.
Markscheme
Anode (negative electrode):
CH3OH(aq) + H2O(l) → 6H+(aq) + 6e− + CO2(g)
Cathode (positive electrode):
O2(g) + 6H+(aq) + 6e− → 3H2O(l)
Award [1 max] for correct equations at wrong electrode.
Accept “e” for “e–”.
Accept “O2(g) + 4H+(aq) + 4e− → 2H2O(l)”.
[2 marks]
allows H+/ions pass through/diffuse/move «from anode to cathode but not electrons or small molecules»
Accept “acts as a salt bridge”.
[1 mark]
H+/ions pass through/diffuse/move from anode/negative electrode «through membrane» to cathode/positive electrode
H+/ions used to reduce oxygen at cathode/positive electrode
Oxygen must be mentioned for M2.
[2 marks]