(a)
Describe what is meant by the wave function of an electron.
[2]
Assess your score
View Answer
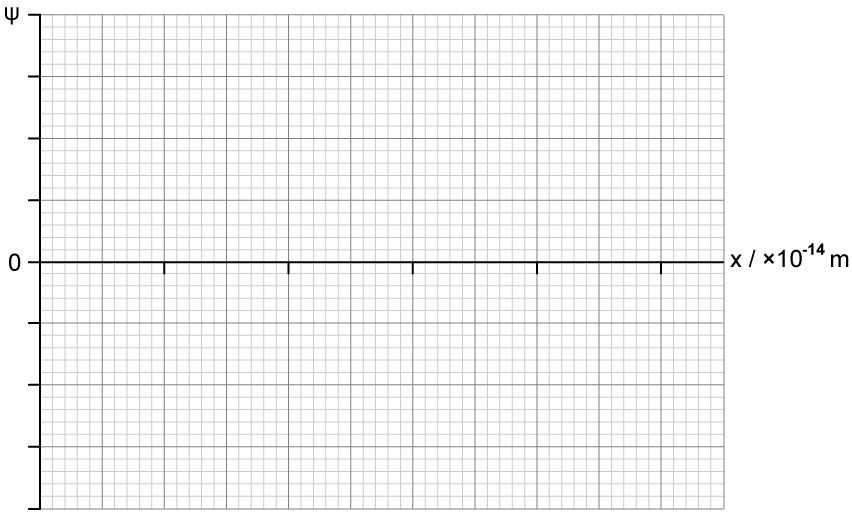
An electron is confined in a finite region of length 6.8 × 10–14 m.
(b)
Determine the uncertainty in the momentum of the electron.
[2]
Assess your score
View Answer
(c)
Determine the associated de Broglie wavelength of the electron if it was accelerated into its confinement through a potential difference of 5.5 GV.
[2]
Assess your score
View Answer
(d)
On the axes provided, sketch the wave function Ψ of the electron described in part (b) and (c) with distance x .
You may assume that Ψ = 0 when x = 0.
[4]
Assess your score
View Answer
Next Question
When monochromatic light is incident on a clean metal surface, photoelectrons may be emitted through the photoelectric effect.
(a)
Outline how Einstein's model is used to explain the photoelectric effect.
[3]
Assess your score
View Answer
(b)
Explain why, although the incident light is monochromatic, the kinetic energies of emitted photoelectrons vary up to some maximum.
[2]
Assess your score
View Answer
(c)
Explain why no photoelectrons are emitted if the frequency of the incident light is less than a certain value, no matter how intense the light.
[2]
Assess your score
View Answer
For monochromatic light of wavelength 570 nm a stopping potential of 1.80 V is required for this particular metal surface.
(d)
Determine the minimum energy required to emit a photoelectron from the metal surface.
[2]
Assess your score
View Answer
Previous Question Next Question
(a)
Outline the de Broglie hypothesis.
[2]
Assess your score
View Answer
(b)
Explain why a precise knowledge of the de Broglie wavelength of an electron implies that its position cannot be measured.
[3]
Assess your score
View Answer
The wave function of Schrödinger's theory can be thought of as a generalisation of the de Broglie hypothesis.
(c)
Outline the relationship between the wave function of Schrödinger's theory and the de Broglie hypothesis.
[3]
Assess your score
View Answer
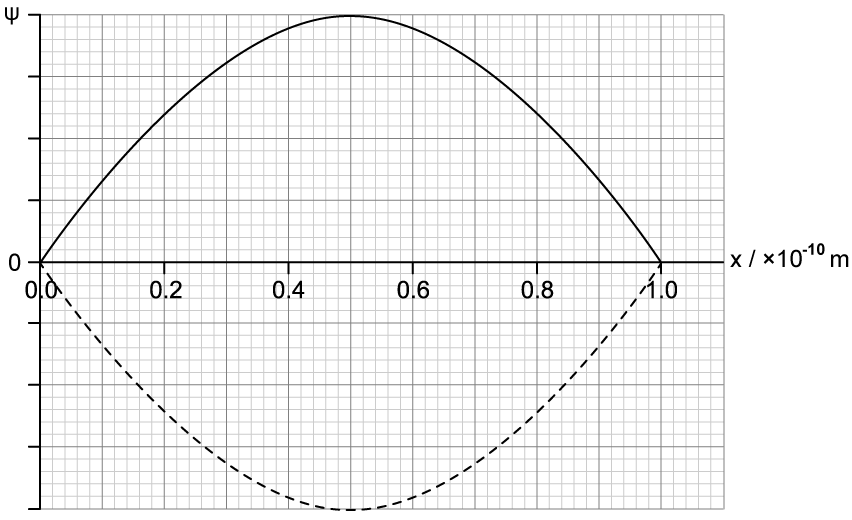
The wave function ψ for an electron confined to length 1.0 × 10–10 m is a standing wave as shown.
(d)
(i)
Explain why the most likely position near which the electron is discoverable is the centre of the box.
[2]
(ii)
Calculate the momentum of the electron.
[2]
Assess your score
View Answer
Previous Question Next Question
One of the striking features of quantum theory is the ability of nature to convert matter into energy and vice versa.
Imagine an electron moving with kinetic energy E k on a collision course with a positron moving in the opposite direction with the same kinetic energy. Following annihilation, two photons are produced.
(a)
Show that the wavelength λ of the two photons produced is given by the expression:
where m e is the mass of the electron.
[4]
Assess your score
View Answer
(b)
Hence, show that the maximum wavelength of photons produced during this annihilation is approximately 2.4 × 10–12 m.
[2]
Assess your score
View Answer
(c)
Show that the minimum wavelength of a photon that can produce an electron–positron pair is approximately 1.2 × 10–12 m.
[2]
Assess your score
View Answer
(d)
Explain why the value for wavelength in part (c) is only an estimate and not an accurate result.
[2]
Assess your score
View Answer
Previous Question Next Question
Monochromatic light is incident on a metal surface and electrons are emitted instantaneously from the surface.
(a)
Explain why:
(i)
electrons are emitted instantaneously.
[2]
(ii)
the energy of the emitted electrons does not depend on the intensity of incident light.
[2]
Assess your score
View Answer
The wavelength of light incident in part (a) is 450 nm and the work function of the metal is 2.0 × 10–19 J.
(b)
Determine the maximum kinetic energy of an electron emitted from the metal surface.
[2]
Assess your score
View Answer
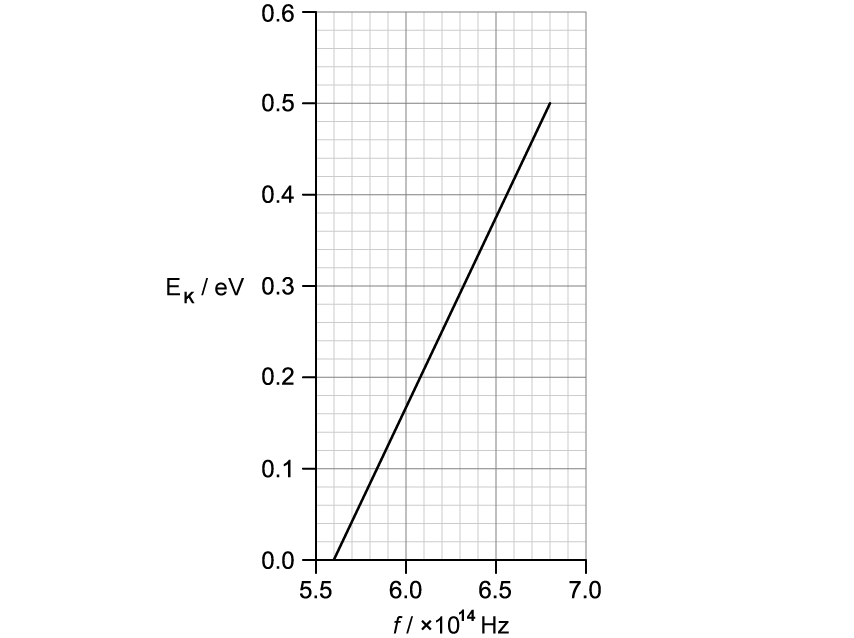
The light source used in part (b) is now incident on a different metal surface. Its frequency is varied, such that the kinetic energy of emitted electrons can be recorded.
The graph shows how the maximum kinetic energy E K of the ejected electrons varies with the frequency of incident light.
(c)
Use the graph to determine a value for the Planck constant h .
[2]
Assess your score
View Answer
(d)
Use the graph in part (c) to determine the work function of the metal.
[2]
Assess your score
View Answer
Previous Question