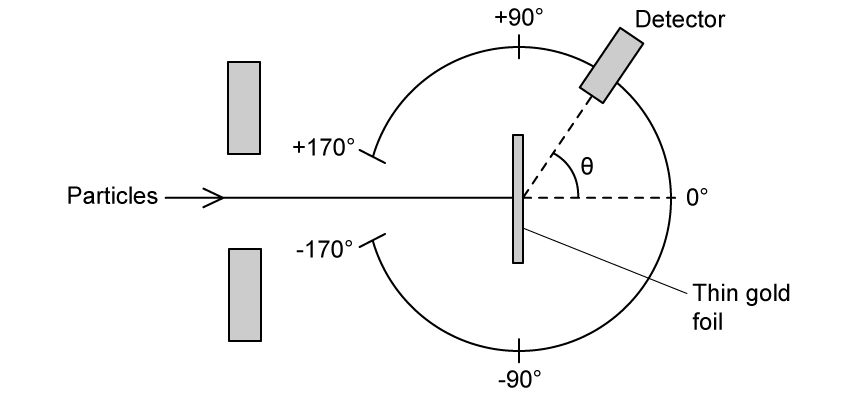(a)
Show that all nuclei have the same density.
[3]
Assess your score
View Answer
A beam of neutrons is fired normally at a thin foil sheet made from tin. The beam has energy 75 MeV and the first diffraction minimum is observed at an angle of 15o relative to the central bright fringe.
(b)
Calculate an estimate for the radius of the tin nucleus.
[4]
Assess your score
View Answer
The tin (
(c)
Deduce and explain the expected difference in the observations between the two experiments.
[2]
Assess your score
View Answer
An isotope of tin has a half-life of 129 days. It undergoes beta-minus decay to a meta-stable isotope of antimony.
(d)
Calculate the percentage of the sample which will consist of antimony after 2 years.
[2]
Assess your score
View Answer
Next Question
Iodine-131
(a)
Calculate the decay constant of
.
[2]
Assess your score
View Answer
The initial activity of the sample of Iodine−131 is 6.5 × 104 Bq.
(b)
Determine the activity after 16 days.
[2]
Assess your score
View Answer
(c)
Determine the mass of the iodine−131 in the sample after 16 days.
[2]
Assess your score
View Answer
Iodine−131 decays through a number of decay modes. Two of these are
(d)
Sketch a nuclear energy level diagram to represent these decays.
[2]
Assess your score
View Answer
Previous Question Next Question
Ernest Rutherford was able to deduce a relationship for the size of the nucleus using the Rutherford scattering experiment shown below:
A radioisotope has a nuclear radius of 7.41 fm
(a)
Determine the nucleon number of the isotope.
[2]
Assess your score
View Answer
A beam of high-energy neutrons is directed at the nucleus and a pattern is formed on a detector screen.
(b)
Explain the pattern which is observed.
[3]
Assess your score
View Answer
The neutrons are accelerated to a speed of 2.88 × 108 m s−1 .
(c)
Determine the angle of the first minimum.
[2]
Assess your score
View Answer
The decay constant of the isotope is 9.72 × 10−10 yr−1 . The mass of a sample of this isotope is 600 g.
(d)
Determine the activity of the sample.
[3]
Assess your score
View Answer
Previous Question Next Question
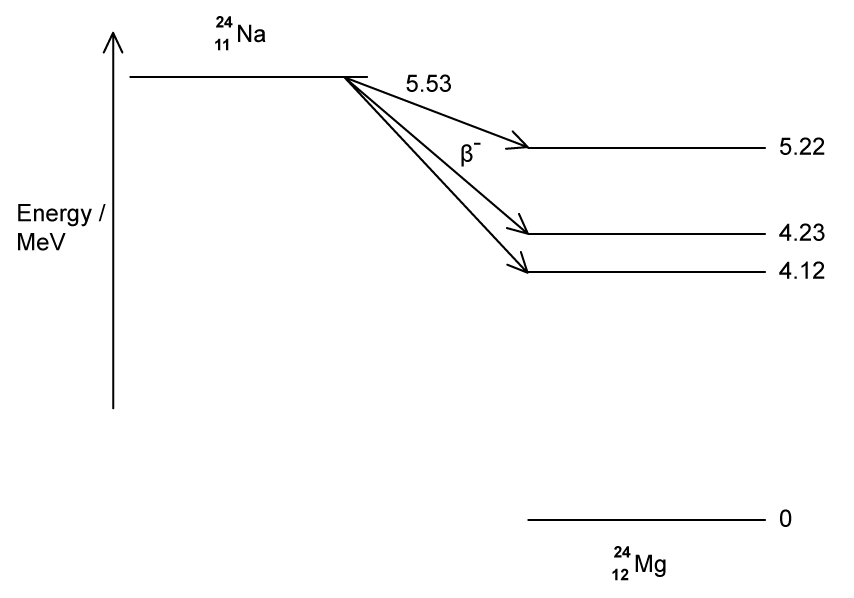
A nucleus of sodium−24 decays into a stable nucleus of magnesium−24. It decays by β – emission followed by the emission of γ-radiation as the magnesium−24 nucleus de-excites into its ground state. The sodium−24 nucleus can decay to one of three excited states of the magnesium−24 nucleus. This is shown in the diagram below:
The energies of the excited states are shown relative to the ground state.
(a)
Calculate the maximum possible speed of the emitted beta particle in MeV.
[2]
Assess your score
View Answer
The excited magnesium nucleus de-excites through production of gamma radiation of discrete wavelengths.
(b)
Calculate the shortest wavelength of emitted radiation.
[3]
Assess your score
View Answer
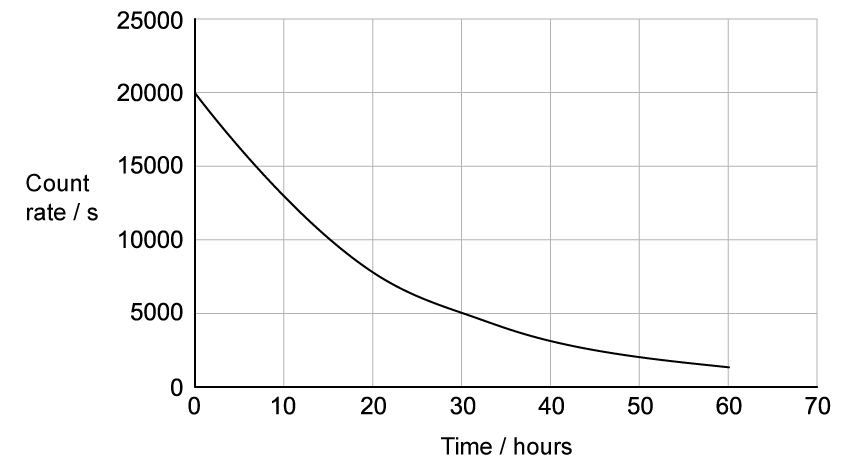
The graph shows the activity of a sample of sodium−24 with time.
(c)
Use the graph to calculate the decay constant of sodium−24.
[2]
Assess your score
View Answer
The detector in this experiment measures 4% of the activity from the sample.
(d)
Determine the activity of sample after 27 hours from the start of the recording,
[3]
Assess your score
View Answer
Previous Question Next Question
Americium-241 has a half-life of 432 years. A small sample is held in a school for use in experiments.
The teacher uses a Geiger-Müller counter to measure the count rate at close range. The relationship between activity and count rate is a ratio of 6:1. Over 5 minutes, the count is 13 600.
(a)
Determine the activity of the sample.
[2]
Assess your score
View Answer
(b)
Determine the activity of the americium sample after 748 years.
[2]
Assess your score
View Answer
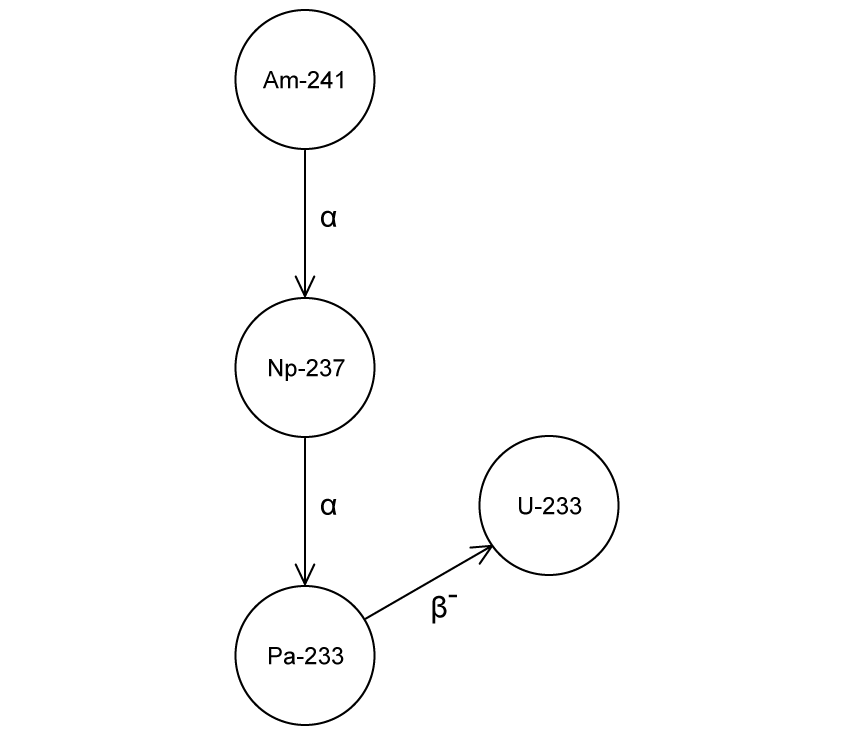
Americium−241 decays through a series of decays to uranium-233.
The energies from each decay path are recorded.
(c)
Explain the differences between the energy profiles for the alpha decays and the beta decays.
[2]
Assess your score
View Answer
The beta decay of protactinium−233 to uranium−233 has a half−life of 27 days. A sample of protactinium has an activity of 3879 Bq.
(d)
Determine the number of protactinium−233 nuclei in the sample.
[2]
Assess your score
View Answer
Previous Question
) foil was replaced by thin aluminium (
) foil.