Question 1b
The unified atomic mass unit (a.m.u) is roughly equal to the mass of one nucleon.
Question 1c
Einstein's Theory of Relativity showed that mass could be converted into energy, and energy into mass. This is summarised in the equation:
The unified atomic mass unit (a.m.u) is roughly equal to the mass of one nucleon.
Einstein's Theory of Relativity showed that mass could be converted into energy, and energy into mass. This is summarised in the equation:
The nuclear rest mass of oxygen−16 is 15.994 914 u.
The mass defect, Δm, equation describes the relationship between the proton number, Z, the number of neutrons, N, the proton rest mass, mp, the neutron rest mass, mn, and the nuclear rest mass, mtotal.
The mass defect (from part (b)) can be used to calculate the binding energy.
The binding energy per nucleon of Helium−4 is 7.1 MeV.
Helium is formed inside main sequence stars due to the process of nuclear ________. For this process to occur, both nuclei must have high _______ energy. This high energy is because the protons inside the nuclei are ________ charged and a great deal of energy is needed to overcome the ________ force of repulsion.
[3]
Nuclear ________ can be induced by firing ________ at a nucleus. When the nucleus is struck it splits into two or more ________ nuclei and more ________. This leads to a chain reaction.
[2]
The chart shows the binding energy per nucleon for a number of nuclei.
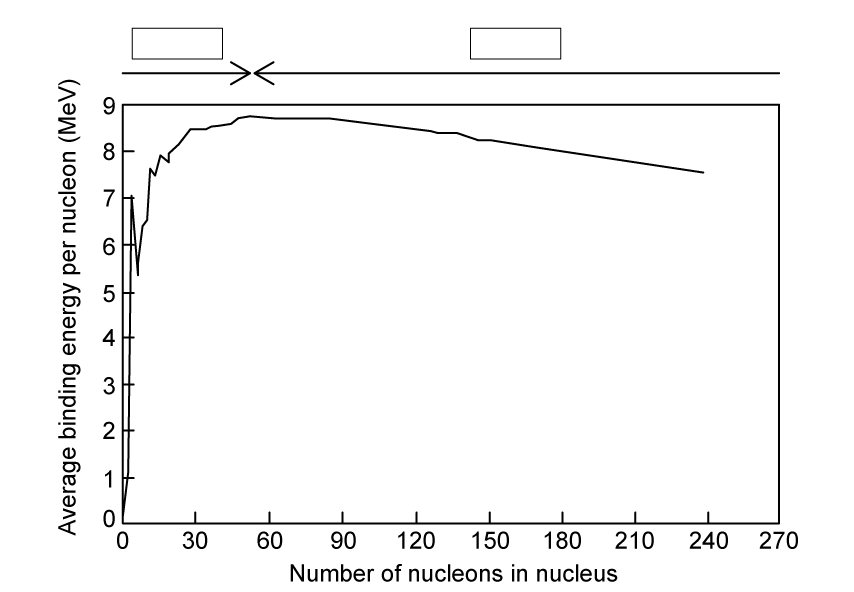
In both fission and fusion, there is a mass defect between the original nuclei and the daughter nuclei.
The graph shows the binding energy per nucleon in MeV plotted against nucleon number, A.
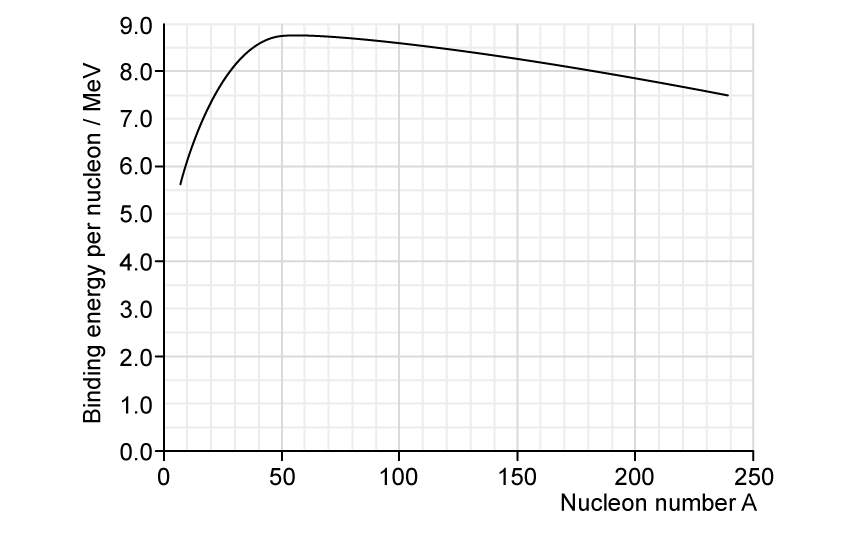
The graph below shows the binding energy per nucleon against the number of nucleons in the nucleus.
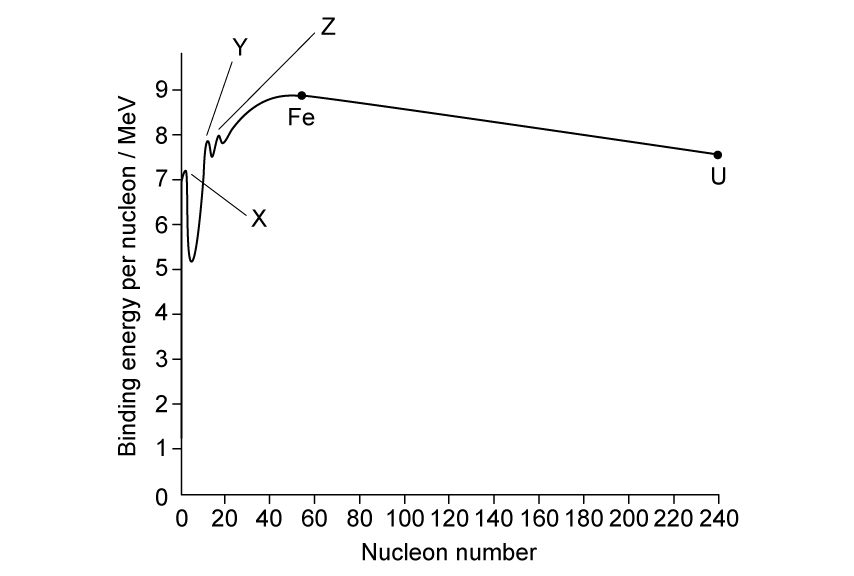
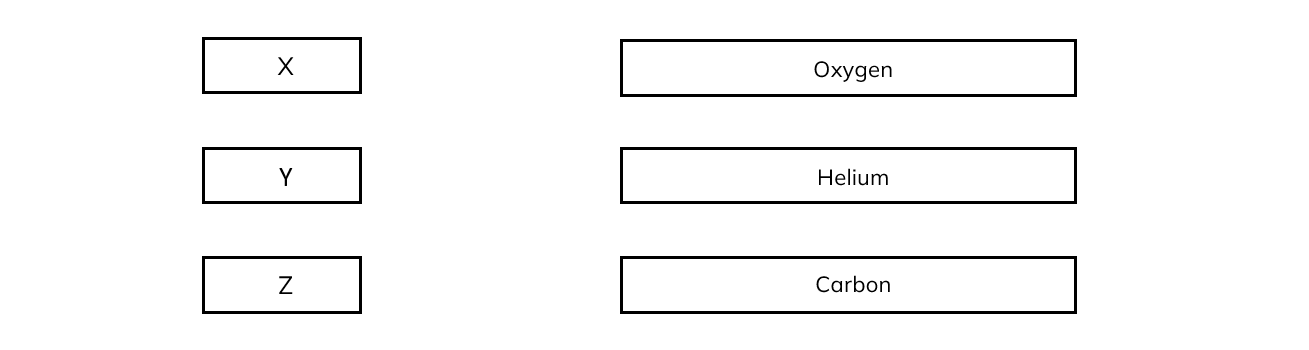
There are three nuclei, labelled X, Y and Z, which do not sit on the line of the graph.
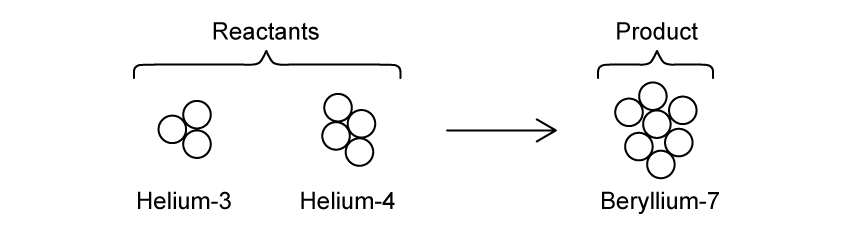
Helium can fuse together to form beryllium as shown in the reaction below:
The table shows the mass of each reactant and daughter nucleus:
Helium−3 and helium−4 fuse together to form beryllium−7.
The mass defect, Δm for this fusion reaction is equal to 2.8 × 10–30 kg.