(a)
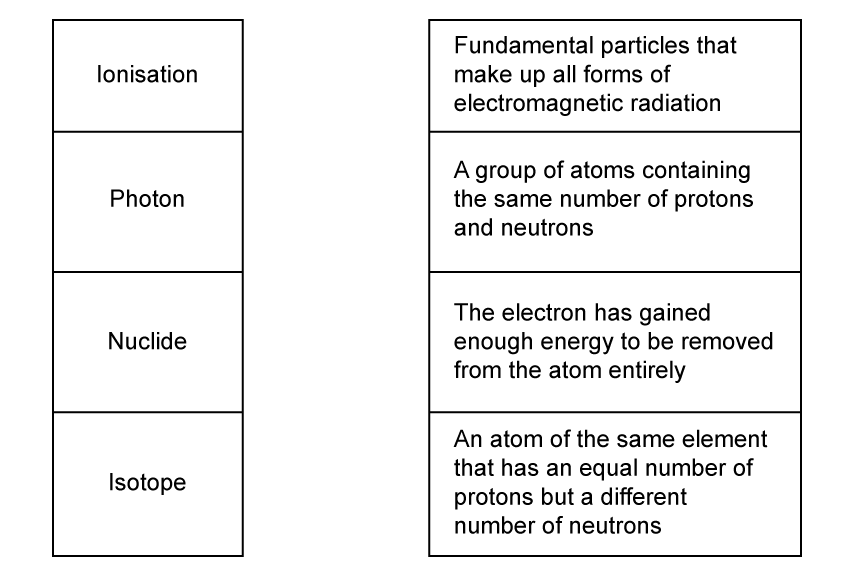
Match, by drawing a line, the words with their correct definitions.
[4]
Assess your score
View Answer
The energy of a photon can be calculated using the equation
(b)
Define the following terms and give the unit:
(i)
h
[2]
(ii)
c
[2]
(iii)
λ
[2]
Assess your score
View Answer
(c)
Calculate the wavelength of a photon with an energy of 1.44 × 10−19 J.
[2]
Assess your score
View Answer
(d)
(i)
Complete the gaps in the following paragraph by writing the correct words on the line.
Electrons in an atom can only have specific energies. These energies are called _________ _________ _______ .
Normally, electrons occupy the _______ energy level available. This is known as the __________ __________.
Electrons can gain energy and move up the energy levels by ______________ energy.
[4]
(ii)
Underline the processes that allow an electron to move up an energy level.
Collisions with other atoms or electrons Releasing a photon Radioactive decay Absorbing a photon Changing colour Emitting a neutrino
A physical source, such as heat
[3]
Assess your score
View Answer
Next Question
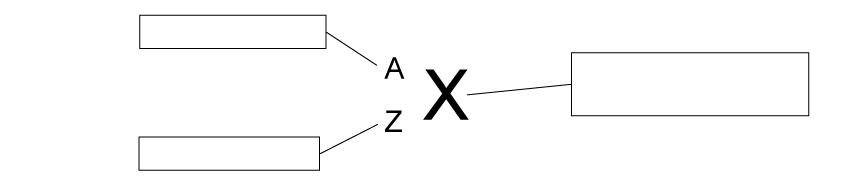
Nuclides can be written in symbol form.
(a)
Complete the labels on the general nuclide symbol using the words below:
Chemical symbol for the element
Proton number Nucleon number
[3]
Assess your score
View Answer
(b)
Define radioactive decay.
[4]
Assess your score
View Answer
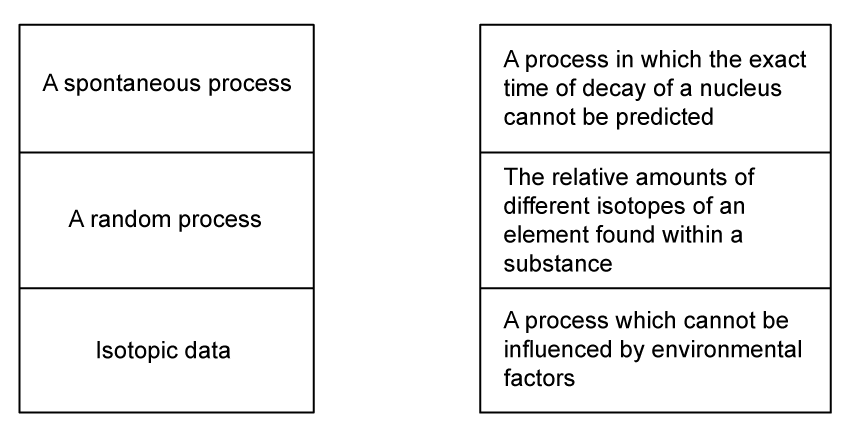
(c)
Draw lines to match the phrases with the correct definitions.
[3]
Assess your score
View Answer
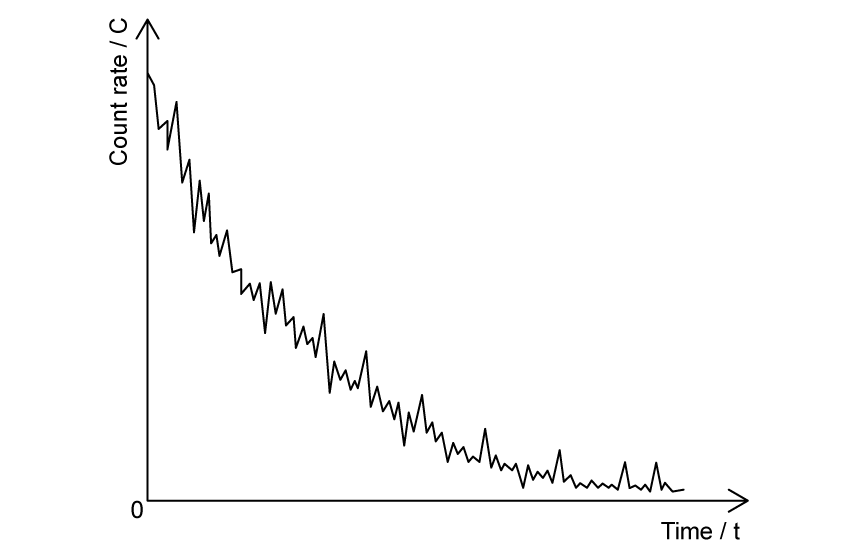
The graph shows the count rate of a radioactive substance measured by a Geiger-Müller tube.
(d)
State what the fluctuations in the count rate provide evidence for.
[1]
Assess your score
View Answer
Previous Question Next Question
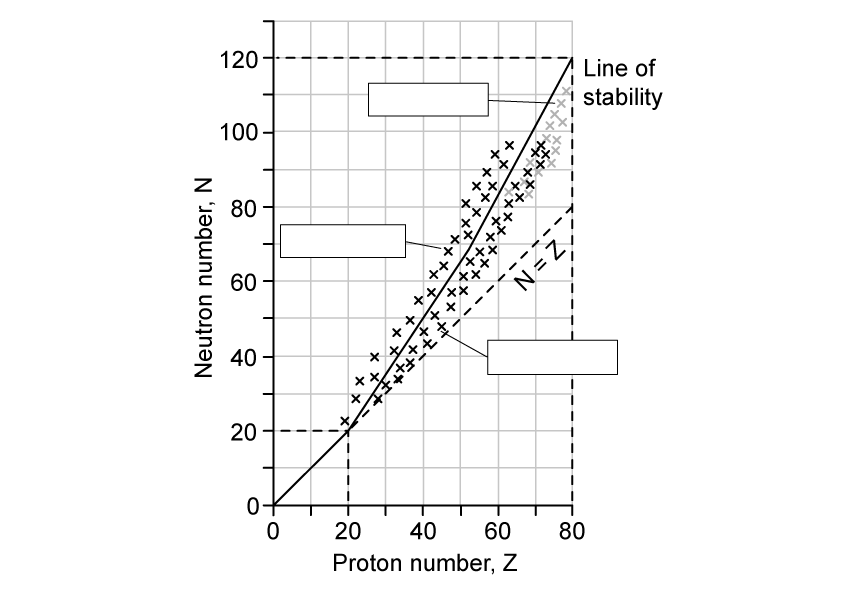
The number of neutrons and number of protons for different isotopes can be plotted on a graph called a nuclear stability curve. Different regions on the graph represent the type of decay which is expected.
The three types of radioactive particles shown are alpha emitters, beta−minus emitters and beta−positive emitters.
(a)
Label the regions of the graph to indicate which type of radioactive particle is expected to be emitted.
[3]
Assess your score
View Answer
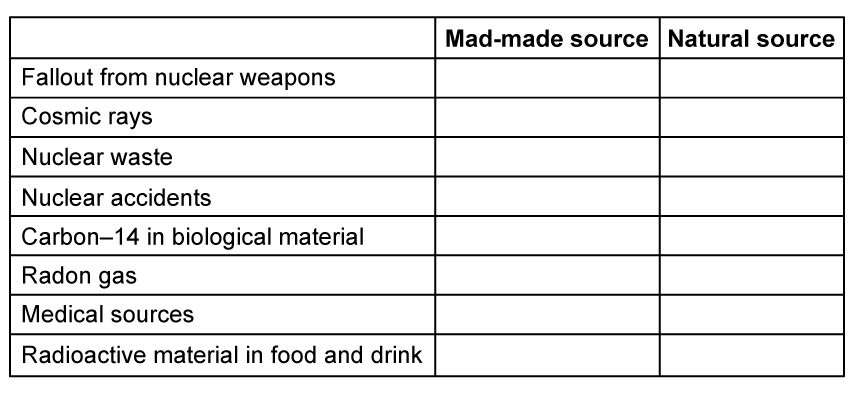
Background radiation comes from a variety of sources, some are natural and some are man-made.
(b)
Place ticks (✔) in the correct column to indicate whether the source is man-made or natural:
[8]
Assess your score
View Answer
Radiation is emitted as various different types of particle.
(c)
State 4 types of radioactive particle.
[4]
Assess your score
View Answer
When a beta emission occurs, a particle called a neutrino is also emitted.
(d)
Complete the gaps in the following sentences. Choose from the words below:
A neutrino has no ___________ and negligable __________. Electron anti−neutrinos are produced during ___________ decay. Electron neutrinos are produced during ___________ decay.
mass gravity age charge beta−minus beta−positive alpha
[4]
Assess your score
View Answer
Previous Question Next Question
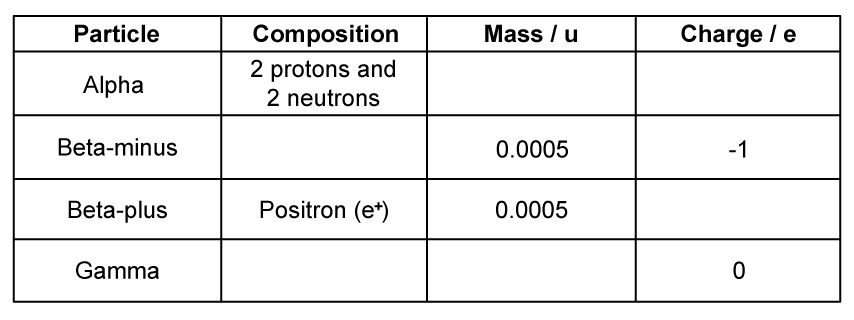
(a)
Complete the table with the correct properties of alpha, beta−minus, beta−positive and gamma radiation.
[6]
Assess your score
View Answer
Plutonium−239 decays to Uranium−235 through the emission of an alpha particle.
(b)
Determine the missing values in the decay equation:
[2]
Assess your score
View Answer
Strontium−90 decays through beta−minus decay to form Yttrium−90.
(c)
Determine the missing values in the decay equation.
[2]
Assess your score
View Answer
Fluorine−18 decays through beta−plus decay to form oxygen−18.
(d)
Determine the missing values in the decay equation.
[2]
Assess your score
View Answer
Previous Question Next Question
(a)
Define half−life.
[2]
Assess your score
View Answer
A student investigates the half−life of technetium with time. This list shows the variables in the experiment.
time size of sample distance from detector to sample
same material for the sample radioactive activity
(b)
Using variables from the list:
(i)
State the independent variable
[1]
(ii)
State the dependent variable
[1]
(iii)
State the control variables for the experiment
[3]
Assess your score
View Answer
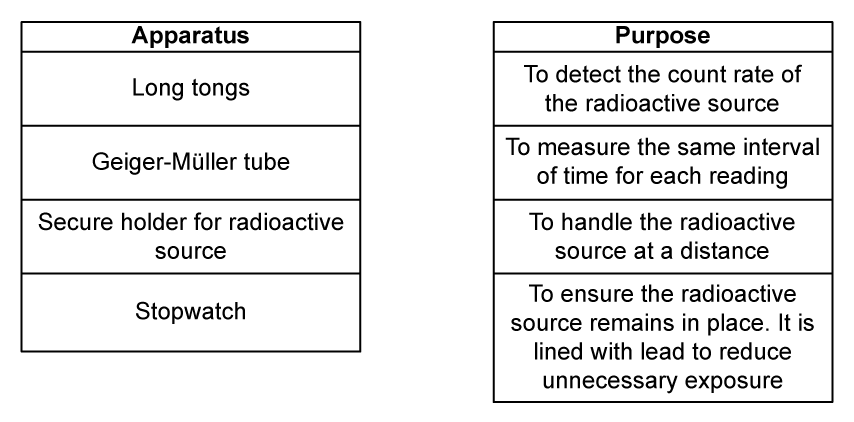
The experiment uses a variety of apparatus.
(c)
Draw a line to match the apparatus with its correct use.
[4]
Assess your score
View Answer
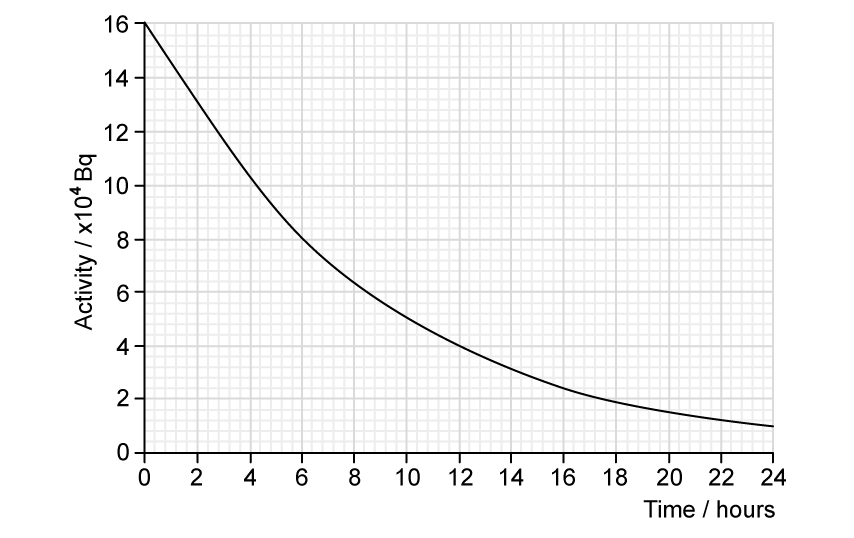
The student plots the following graph of their results.
(d)
Determine the half−life of the sample.
[3]
Assess your score
View Answer
Previous Question