Which of the statements is the correct definition of excitation of an electron?
When an electron is removed from or added to an atom
When an electron moves down an energy level emitting a photon
When an electron is given enough energy to move up an energy level, but not enough to leave the atom
When an electron is given enough energy to move up an energy level, and sometimes leave the atom
The diagram below shows the energy levels of a mercury atom.
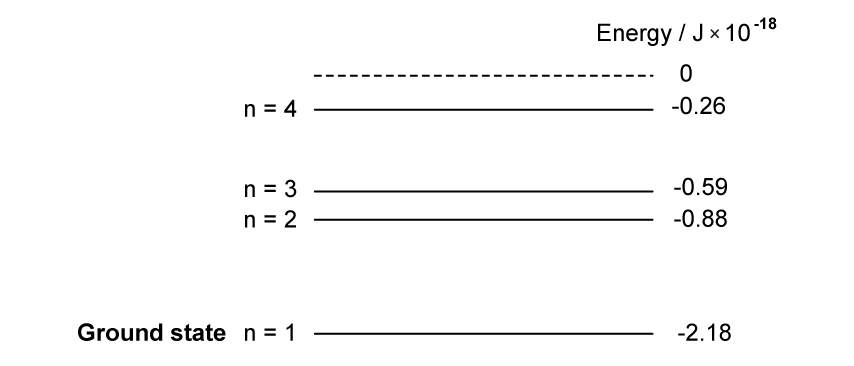
Which transition produces a photon with the longest wavelength?
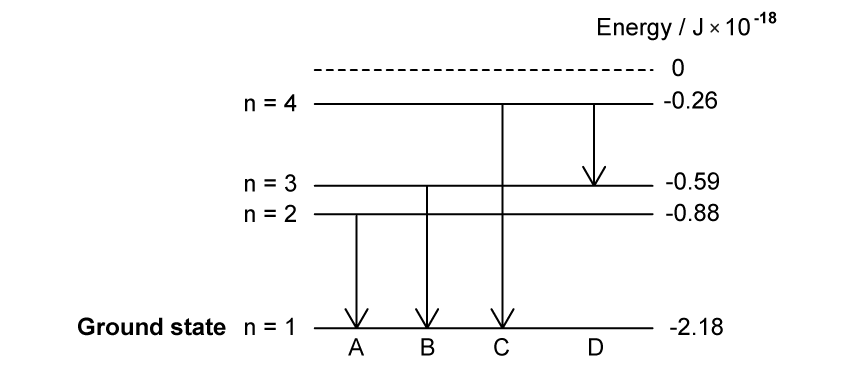
The diagram shows an energy–level diagram for a hydrogen atom.
How many discrete photon energies could be produced from these energy levels?
Which of the following is not a feature of an emission spectrum?
When an electron transitions from a higher energy level to a lower energy level, this results in the emission of a photon
An emission spectrum contains a set of discrete wavelengths, represented by coloured lines on a black background
An emission spectrum is evidence to show that electrons in atoms can only transition between discrete energy levels
An emission spectrum consists of a continuous spectrum containing all the colours with dark lines at certain wavelengths
A larger version of the hydrogen emission spectrum from the infrared to the ultraviolet region looks as follows:
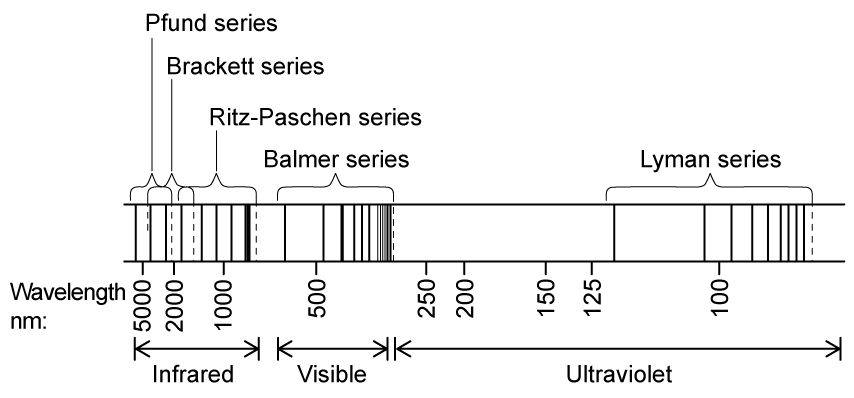
Using information in the diagram, which hydrogen series corresponds to the highest energy photons being emitted?
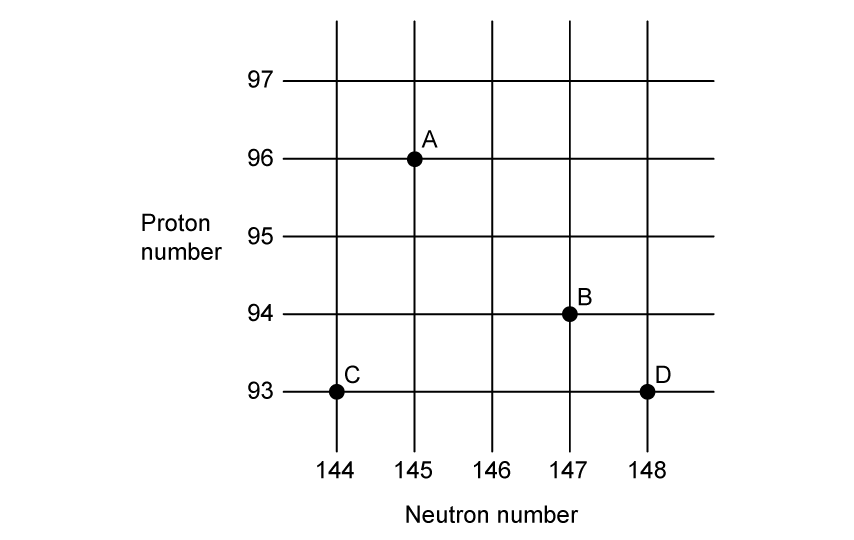
An isotope of  undergoes alpha-decay.
undergoes alpha-decay.
Which letter on the N-Z graph below represents the correct proton-neutron product of the decay?
Uranium−238 undergoes radioactive decay to become Thorium−234. A second decay occurs and the product is protactinium−234.
The decay sequence can be represented in equation form, where i, j and k are particles.:
format('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2213.5%22%20y%3D%2226%22%3E92%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2210.5%22%20y%3D%2212%22%3E238%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2218%22%20text-anchor%3D%22middle%22%20x%3D%2227.5%22%20y%3D%2217%22%3EU%3C%2Ftext%3E%3Ctext%20font-family%3D%22math1694be756a8e86d4bc848e34630%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%2244.5%22%20y%3D%2217%22%3E%26%23x2192%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2267.5%22%20y%3D%2226%22%3E90%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2264.5%22%20y%3D%2212%22%3E234%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2218%22%20text-anchor%3D%22middle%22%20x%3D%2285.5%22%20y%3D%2217%22%3ETh%3C%2Ftext%3E%3Ctext%20font-family%3D%22math1694be756a8e86d4bc848e34630%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%22107.5%22%20y%3D%2217%22%3E%2B%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2218%22%20font-style%3D%22italic%22%20text-anchor%3D%22middle%22%20x%3D%22122.5%22%20y%3D%2217%22%3Ei%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2213.5%22%20y%3D%2281%22%3E90%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2210.5%22%20y%3D%2267%22%3E234%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2218%22%20text-anchor%3D%22middle%22%20x%3D%2231.5%22%20y%3D%2272%22%3ETh%3C%2Ftext%3E%3Ctext%20font-family%3D%22math1694be756a8e86d4bc848e34630%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%2251.5%22%20y%3D%2272%22%3E%26%23x2192%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2274.5%22%20y%3D%2281%22%3E91%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2213%22%20text-anchor%3D%22middle%22%20x%3D%2271.5%22%20y%3D%2267%22%3E234%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2218%22%20text-anchor%3D%22middle%22%20x%3D%2291.5%22%20y%3D%2272%22%3EPa%3C%2Ftext%3E%3Ctext%20font-family%3D%22math1694be756a8e86d4bc848e34630%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%22112.5%22%20y%3D%2272%22%3E%2B%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2218%22%20font-style%3D%22italic%22%20text-anchor%3D%22middle%22%20x%3D%22127.5%22%20y%3D%2272%22%3Ej%3C%2Ftext%3E%3Ctext%20font-family%3D%22math1694be756a8e86d4bc848e34630%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%22143.5%22%20y%3D%2272%22%3E%2B%3C%2Ftext%3E%3Ctext%20font-family%3D%22Times%20New%20Roman%22%20font-size%3D%2218%22%20font-style%3D%22italic%22%20text-anchor%3D%22middle%22%20x%3D%22160.5%22%20y%3D%2272%22%3Ek%3C%2Ftext%3E%3C%2Fsvg%3E)
What are the correct names of these particles?
| |
i
|
j
|
k
|
|
A
|
alpha
|
beta−minus
|
electron anti−neutrino
|
|
B
|
beta−positive
|
beta−minus
|
alpha
|
|
C
|
beta−minus
|
neutron
|
photon
|
|
D
|
alpha
|
beta−positive
|
neutron
|
What are the correct numbers of protons, neutrons and electrons in a neutral atom of  ?
?
| |
protons
|
neutrons
|
electrons
|
|
A
|
92
|
92
|
92
|
|
B
|
92
|
142
|
92
|
|
C
|
142
|
92
|
142
|
|
D
|
234
|
142
|
92
|
What is the charge on, and mass of, an electron neutrino and during what process is an electron neutrino produced?
| |
charge /e
|
mass / u
|
production of neutrino
|
|
A
|
+1
|
zero
|
during β+ emission
|
|
B
|
−1
|
+1
|
during β− emission
|
|
C
|
zero
|
zero
|
during β+ emission
|
|
D
|
zero
|
0.0005
|
during β− emission |
A sample of californium−239 has an activity of 4000 Bq. The half−life of californium−239 is 1 minute.
What will the activity be after 4 minutes?




