Question 1
A subatomic particle of mass m has an uncertainty in its position r, denoted by Δr. What is the uncertainty in its velocity, Δv?
A subatomic particle of mass m has an uncertainty in its position r, denoted by Δr. What is the uncertainty in its velocity, Δv?
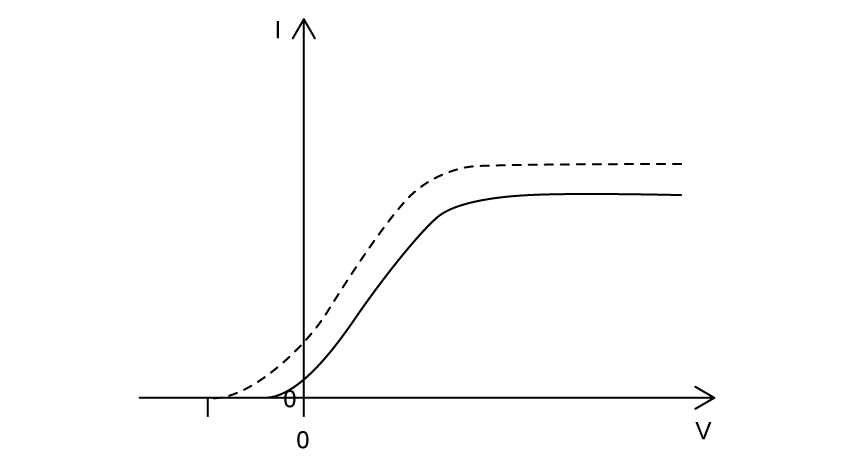
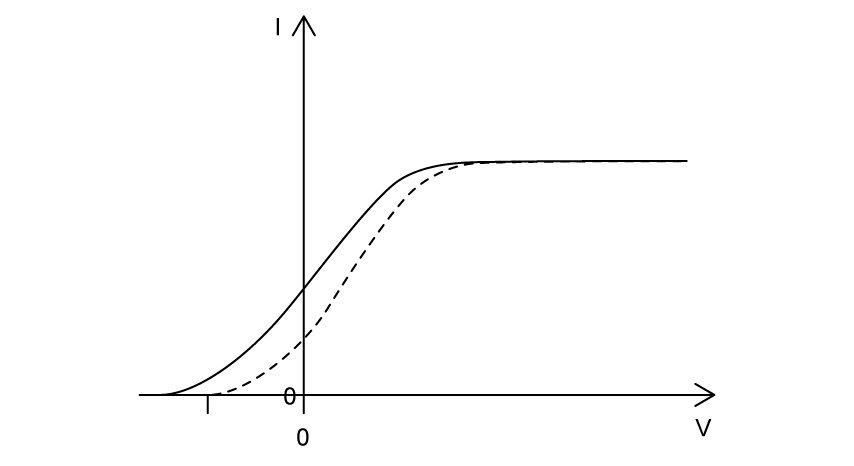
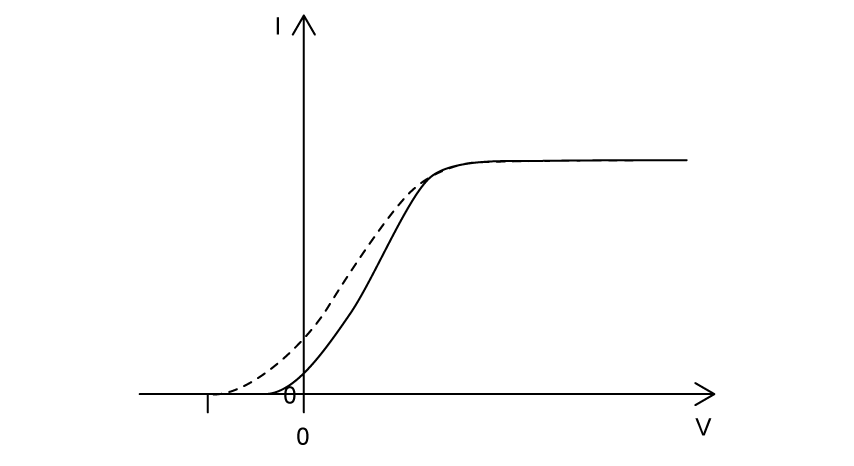
Violet light is incident on a metal surface, producing photoelectrons.
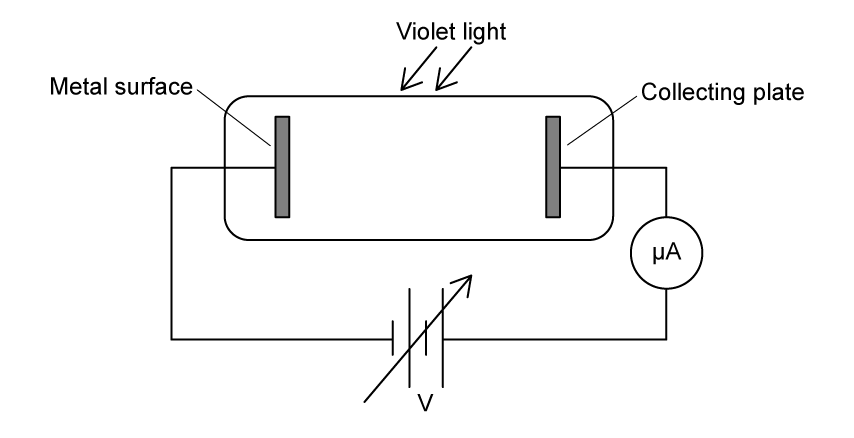
The variation of photocurrent I with potential difference V is shown.
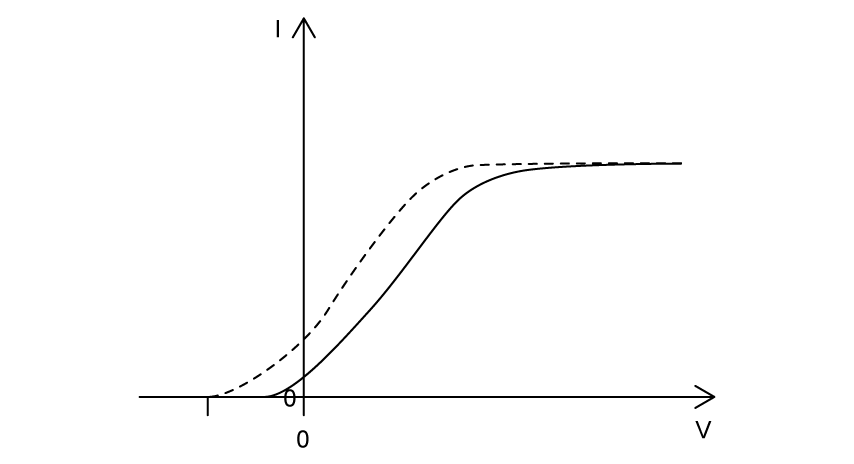
The light source is changed to red light of the same intensity as the violet light. Which graph shows the variation of photocurrent I with potential difference V for the red light? The results for the violet light are shown as a dashed line.
Which expression is proportional to the probability of finding an electron in a particular region of space?
The magnitude of the wave function
The square of the magnitude of the wave function
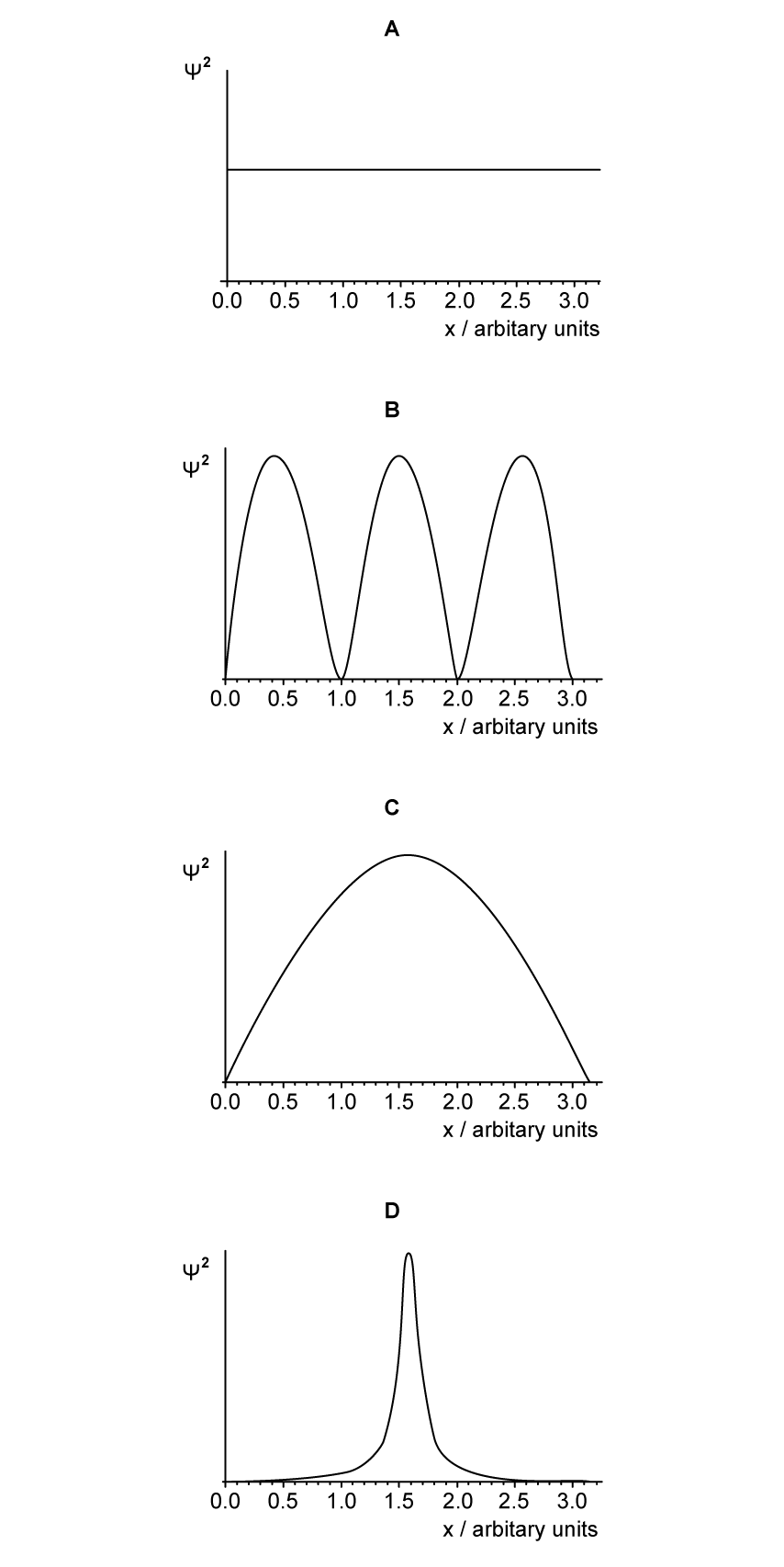
The graphs show the variation with distance of the square of the magnitude of the wave function,
, of a particle. Which graph corresponds to a particle with the largest uncertainty in momentum?
The graph shows how the wave function Ψ of an electron varies with distance x.
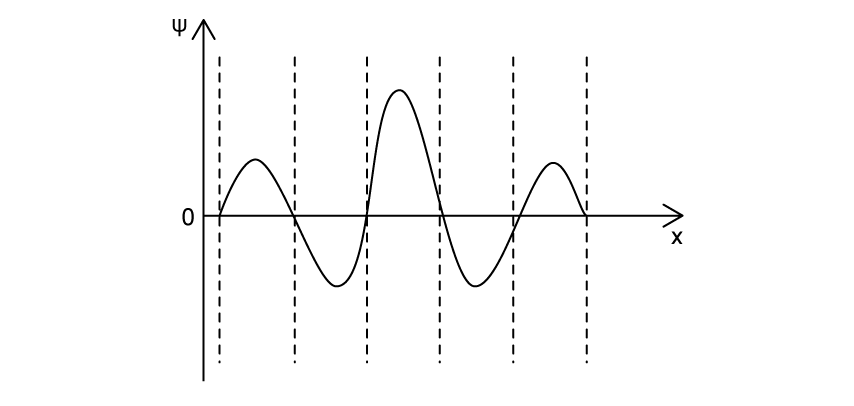
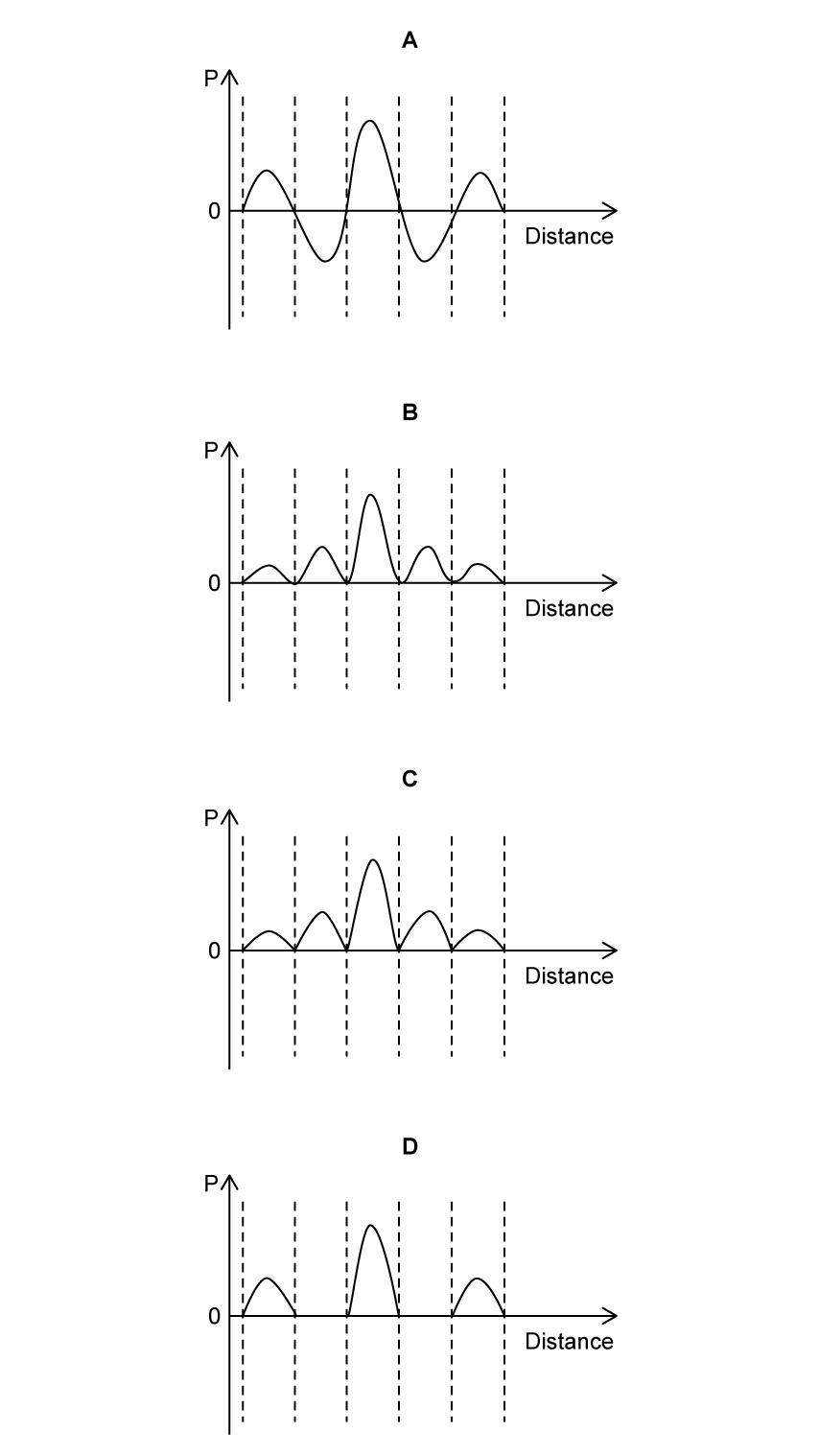
Which of the following graphs shows the probability of finding the electron at each position along the distance x?
According to Heisenberg's uncertainty principle, conjugate quantities are pairs of quantities that cannot be known simultaneously with unlimited precision. What unit represents the product of two conjugate quantities?
kg2 m s–1
kg m2 s
kg m2 s–1
kg m2 s–2
Alpha particles of mass m are accelerated from rest through a potential difference ΔV. Which of the following gives the de Broglie wavelength of the alpha particles as a result of the acceleration?
Use the following data:
Which expression evaluates the de Broglie wavelength of an electron of mass m and charge e in the n = 2 state of hydrogen?
The electron wave function Ψ is a function of position and time. Which expression evaluates the probability of discovering the electron in some volume ΔV?
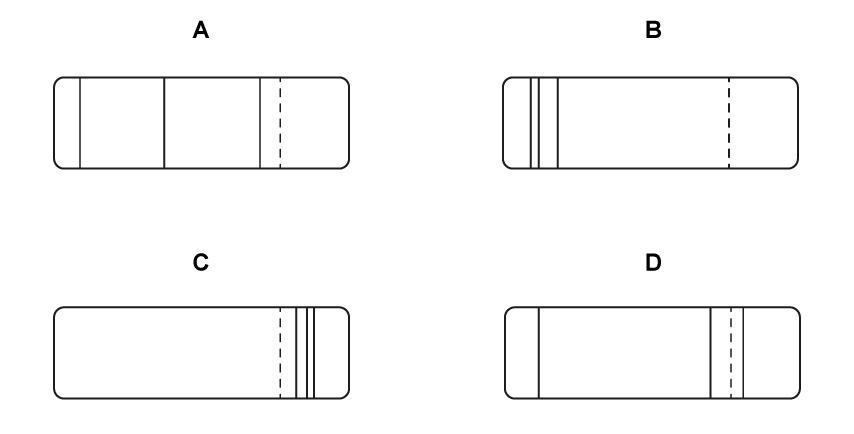
According to the Bohr model for hydrogen, visible light is emitted when electrons make transitions from excited states down to the state with n = 2.
The dotted line in the diagram represents such a transition, from n = 3 to n = 2, in the spectrum of hydrogen.
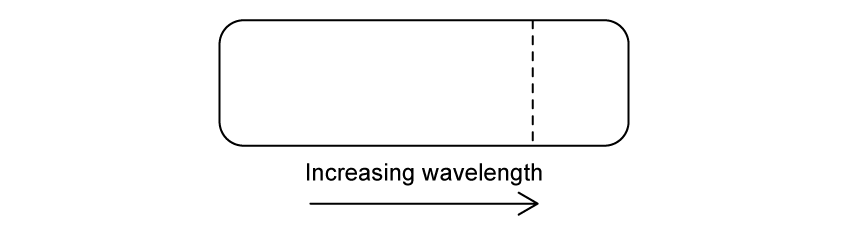
Which of the following diagrams could represent the visible light emission spectrum of hydrogen?