But-1-ene reacts separately with HBr and H2/ Ni to give products X and Z respectively.
What are the major products of the reactions?
| |
X |
Z |
| A. |
CH2BrCH2CH2CH3 |
CH3CH2Cformat('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22math197ea3cfb64eeaa1edba65501d0%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%228.5%22%20y%3D%2216%22%3E%26%23x2261%3B%3C%2Ftext%3E%3C%2Fsvg%3E) CH CH |
| B. |
CH3CH2CH2CH2OH |
CH3CH2CH2CH3 |
| C. |
CH3CHBrCH2CH3 |
CH3CH2Cformat('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22math197ea3cfb64eeaa1edba65501d0%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%228.5%22%20y%3D%2216%22%3E%26%23x2261%3B%3C%2Ftext%3E%3C%2Fsvg%3E) CH CH |
| D. |
CH3CHBrCH2CH3 |
CH3CH2CH2CH3 |
Methylbenzene can be converted into 4-methylphenylamine in a multi-step reaction.
Which order should the reagents be used to do this conversion?
| |
1st reagent |
2nd reagent |
3rd reagent |
| A. |
conc. HNO3 / conc. H2SO4 |
Sn / conc. HCl |
NaOH |
| B. |
Sn / conc. HCl |
conc. HNO3 / conc. H2SO4 |
NaOH |
| C. |
Sn / conc. HCl |
NaOH |
conc. HNO3 / conc. H2SO4 |
| D. |
NaOH |
conc. HNO3 / conc. H2SO4 |
Sn / conc. HCl |
Which alcohol could not be produced by the reduction of an aldehyde or a ketone?
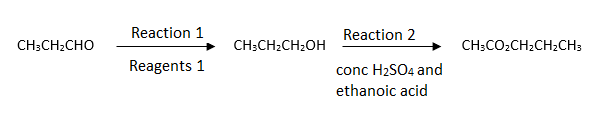 What are the reagents used and the reactions taking place?
What are the reagents used and the reactions taking place?