Question 1
Which species can be represented with only one Lewis structure?
H2PO4-
O3
CH3COCH3
1,3 cyclohexadiene
Which species can be represented with only one Lewis structure?
H2PO4-
O3
CH3COCH3
1,3 cyclohexadiene
In which group do both compounds contain delocalised electrons?
K2CO3 and cyclohexene, C6H10
KOH and methybenzene, C7H8
KHCO3 and buta-1,3-diene, C4H6
Methanol and sodium methanoate, HCOONa
Which of the following are intermediate species in the catalytic depletion of ozone?
I. ClO•
II. NO•
III. NO2•
I and II only
I and III only
II only
I, II and III
Which is not a correct statement about sp3 hybridised carbons?
They can form sigma or pi bonds
They have the electron arrangement 1s22s12p3
They have four bonding orbitals of equal energy
The bonding orbitals have s character and
p character
The structure of the painkiller paracetamol, often known as acetaminophen, is shown below:
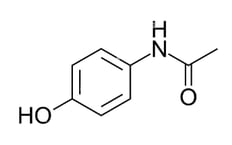
Which of the following is not present in the structure?
5 lone pairs
7 atoms with bond angles of 120o around them
8 atoms with sp2 hybrid orbitals
16 sigma (σ) bonds