a)
On the axes below, draw a sketch graph to show the neutralisation of ethanoic acid by sodium hydroxide:
[2]
b)
Write an equation for the reaction between ethanoic acid and sodium hydroxide and identify the species acting as a Lewis base in the reaction.
[2]
c)
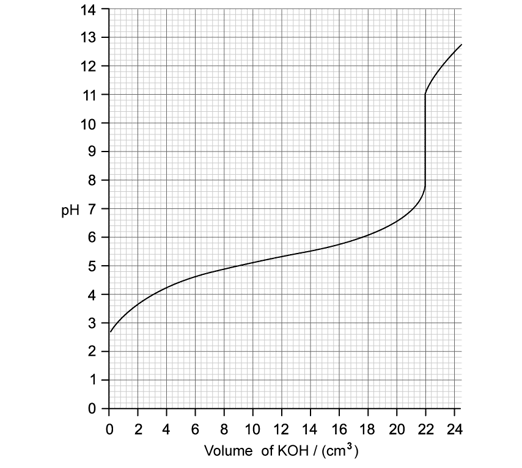
Identify the type of titration taking place from the curve and indicate where the buffer region is found on this curve.
d)
Identity on the graph the point at which p
Ka= pH and find the p
Ka of the acid.
[2]
a)
Explain how an acid-base indicator works.
[3]
b)
Phenolphthalein, C20H14O4, is an acid-base indicator. State the formula and colour of the conjugate base of phenolphthalein.
[2]
c)
Explain how suitable indicators are chosen for titrations.
[3]
a)
Outline what is meant by a buffer solution.
[1]
b)
Outline how a buffer solution can be made starting from 1.0 mol dm-3 ethanoic acid and 1.0 mol dm-3 sodium hydroxide.
[2]
c)
Use suitable equations to explain how the buffer in b) functions when a small quantity of acid is added.
[4]
d)
State the composition of a basic buffer.
[1]