a)
Dichloromethyl benzene reacts with chlorine to produce trichloromethyl benzene. State the name of this type of menchanism and the required condition.
[2]
Assess your score
View Answer
b)
Outline the mechanism for the reaction occurring in part a).
Assess your score
View Answer
c)
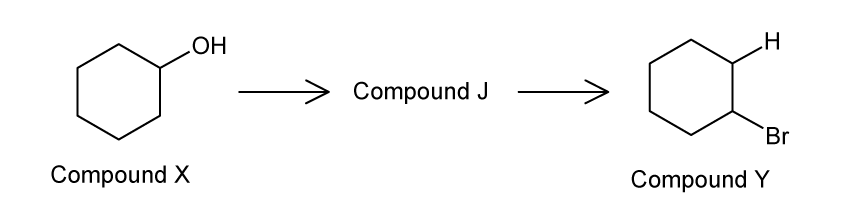
A reaction pathway is shown below . Compound J reacts with bromine water to form a colourless solution.
[1]
Assess your score
View Answer
d)
Identify the reagents and conditions for the formation of Compound Y from Compound J.
[2]
Assess your score
View Answer
Next Question
a)
Compounds W , X and Y are all carbohydrates with X and Y each containing five carbons. Compound W and a byproduct, compound Z , are formed from the reaction between compound X and Y . Compound X can be oxidised by the reaction with acidified potassium dichromate to give compound Y .
Y contains 0.027 moles.
Y and justify your answer.
[3]
Assess your score
View Answer
b)
Deduce the structural formula of compound W and explain how compound Z is formed in the reaction.
[2]
Assess your score
View Answer
c)
Compound X will oxidise to compound Y if allowed to fully oxidise. Explain how a student could stop the full oxidation of compound X .
[4]
Assess your score
View Answer
d)
Deduce the formula of an isomer of compound X that will not react with acidified potassium dichromate, H + / K 2 Cr 2 O 7 .
[1]
Assess your score
View Answer
Previous Question Next Question
a)
Ester A is responsible for a raspberry scent and has the molecular formula C 5 H 10 O 2 . Ester A can be produced by the reaction of an acid with a branched primary alcohol. Identify the acid and alcohol used to prepare ester A .
[2]
Assess your score
View Answer
b)
State the IUPAC name and draw the structural formula of ester A .
[2]
Assess your score
View Answer
c)
State the name of the product when the alcohol used to form ester A reacts with potassium permanganate, KMnO4 (aq).
[1]
Assess your score
View Answer
Previous Question Next Question
a)
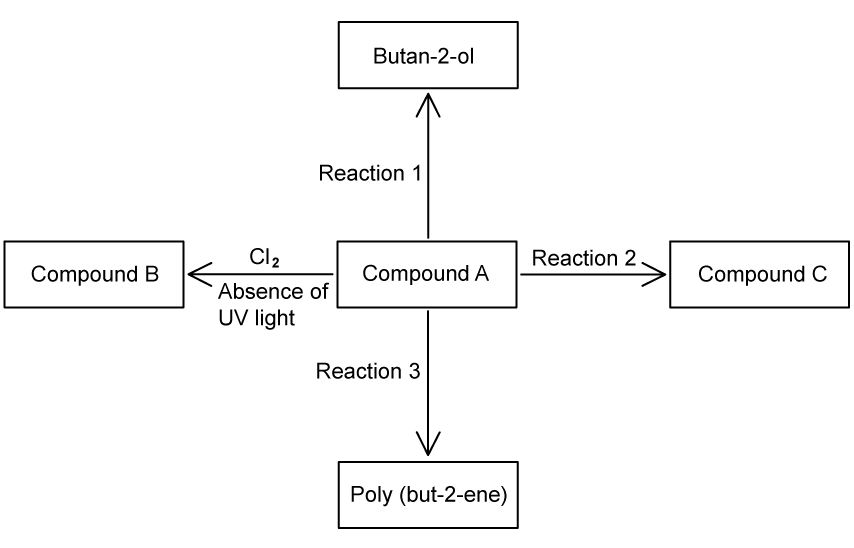
The following scheme shows reactions of Compound A.
i)
Deduce the structural formula of compound A .
[1]
ii)
Apply IUPAC rules to name compound B .
[1]
Assess your score
View Answer
b)
Reaction 1 forms an alcohol when reacted with concentrated sulfuric acid, H2 SO4 and steam.
i)
State the conditions required for this reaction.
[1]
ii)
Deduce the structure of the intermediate in this reaction.
[1]
Assess your score
View Answer
c)
Butan-2-ol can also be directly formed from a halogenoalkane.
i)
State the name of of the type of reaction occurring in this conversation.
[2]
ii)
State the conditions for this reaction.
[1]
Assess your score
View Answer
d)
Identify the structure of the repeating unit of poly(but-2-ene).
[1]
Assess your score
View Answer
e)
Compound A reacts with hydrogen bromide to form compound C. A student suggested a possible formula of compound C is CH2 (Br)CH2 CH2 CH3 .
State whether the student is correct and justify your answer.
[1]
Assess your score
View Answer
Previous Question Next Question
a)
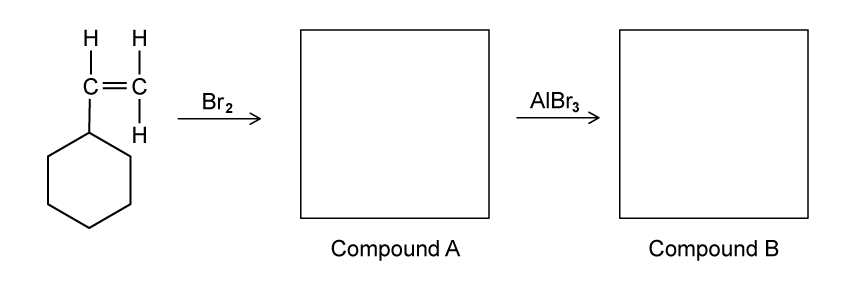
A student investigated two reactions of phenylethene, C6 H5 CHCH2 . First she reacted phenylethene with excess bromine at room temperature to form Compound A . She then added aluminium bromide, AlBr3 to the reaction mixture to form Compound B .
Draw the structure of Compound A and identify one the isomers of C8 H7 Br3 formed in the second reaction.
Assess your score
View Answer
b)
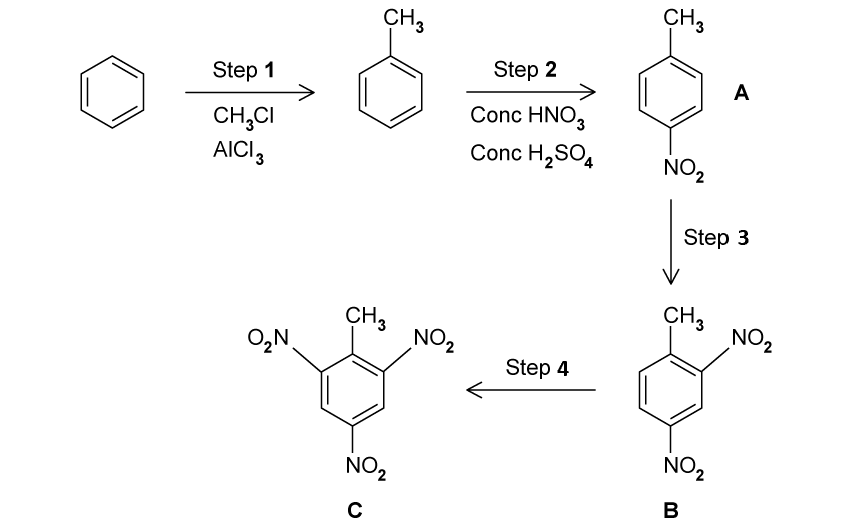
2,4,6-trinitrotoluene (TNT) can be manufactured from benzene as shown below.
5.00 g of benzene was used in step 1. Use section 6 of the data booklet to determine the theoretical yield for step 1.
[2]
Assess your score
View Answer
c)
Step 2 involves the formation of a nitronium ion for the nitration of Toluene, as shown in the following equation:
HNO 3 + 2H 2 SO 4 → NO2 + + 2HSO 4 - + H 3 O +
i)
Explain the role of the nitric acid in the formation of the electrophile.
[2]
ii)
Explain the role of the sulphuric acid in the overall nitration reaction.
[1]
Assess your score
View Answer
d)
Explain why the product of step 2 is most likely to have the nitro group bonded to the second or fourth carbon atom.
[1]
Assess your score
View Answer
Previous Question