Question 1a
a)
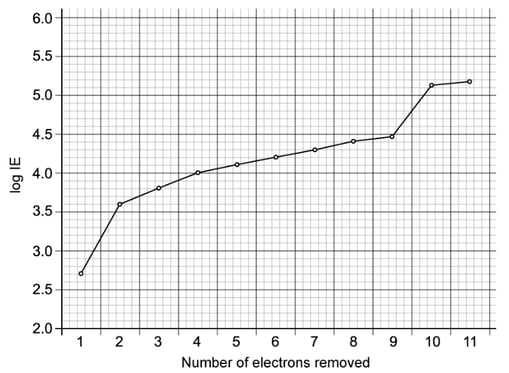
An element Y has the following first six ionisation energies in kJ mol-1. These are shown in the table below.
|
1st |
2nd |
3rd |
4th |
5th |
6th |
|
|
Ionisation energy (kJ mol-1) |
577 |
1820 |
2740 |
11600 |
14800 |
18400 |
State what group of the Periodic Table this element belongs to.
[1]
Question 1b
b)
State what can be determined from the frequency of the convergence limit in a hydrogen emission spectrum.
[1]
Question 1c
c)
Hydrogen spectral data give the frequency of 3.30 x 1015 Hz for its convergence limit.
Calculate the ionisation energy, in J, for a single atom of hydrogen using Sections 1 and 2 of the Data Booklet.
[1]
Question 1d
d)
Calculate the wavelength, in m, for the electron transition corresponding to the frequency in part (c) using Section 1 of the Data Booklet.