a)
Propan-1-ol can be synthesised from alkene P in the following synthetic route:
alkene P format('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22Arial%22%20font-size%3D%2210%22%20text-anchor%3D%22middle%22%20x%3D%2218.5%22%20y%3D%2212%22%3EStep%3C%2Ftext%3E%3Ctext%20font-family%3D%22Arial%22%20font-size%3D%2210%22%20text-anchor%3D%22middle%22%20x%3D%2235.5%22%20y%3D%2212%22%3E1%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%223.5%22%20y%3D%2223%22%3E%26%23xF20B%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%228.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2213.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2218.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2223.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2228.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2233.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2238.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2243.5%22%20y%3D%2223%22%3E%26%23xF200%3B%3C%2Ftext%3E%3C%2Fsvg%3E)
![]()
![]()
![]()
![]()
![]()
![]() halogenoalkane Q
halogenoalkane Q format('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22Arial%22%20font-size%3D%2210%22%20text-anchor%3D%22middle%22%20x%3D%2218.5%22%20y%3D%2212%22%3EStep%3C%2Ftext%3E%3Ctext%20font-family%3D%22Arial%22%20font-size%3D%2210%22%20text-anchor%3D%22middle%22%20x%3D%2235.5%22%20y%3D%2212%22%3E2%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%223.5%22%20y%3D%2223%22%3E%26%23xF20B%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%228.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2213.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2218.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2223.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2228.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2233.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2238.5%22%20y%3D%2223%22%3E%26%23x23AF%3B%3C%2Ftext%3E%3Ctext%20font-family%3D%22horizontal225a4f6397f19c438fc1d%22%20font-size%3D%2214%22%20text-anchor%3D%22middle%22%20x%3D%2243.5%22%20y%3D%2223%22%3E%26%23xF200%3B%3C%2Ftext%3E%3C%2Fsvg%3E)
![]() propan-1-ol
propan-1-ol
i)
State the identity of halogenoalkane Q.
[1]
ii)
Give the reagents and conditions needed for Step 2.
[2]
b) Give the name and structure of alkene P.
c)
Give a reagent that could be used to convert P to Q and outline why this synthesis of propan-1-ol might not be very efficient.
[3]
d)
This question is about alkene P and Step 1.
i)
Give the empirical formula of P.
[1]
ii)
Give the reagents and conditions needed for Step 1.
[2]
iii)
State the type of reaction mechanism.
[1]
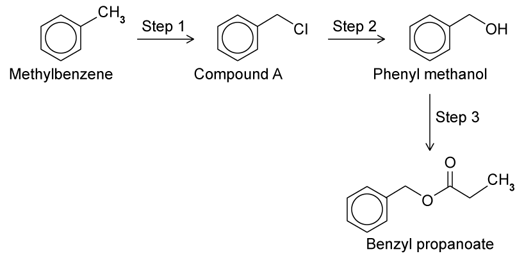
a)
A three step synthesis of benzyl propanoate is shown below:
i)
Give the reagents and conditions needed for Step 1.
[2]
ii)
Name the type of reaction mechanism taking place in Step 1.
[1]
b)
This question is about Step 2.
i)
Give the reagents and conditions needed for Step 2.
[2]
ii)
Name the type of mechanism taking place.
[1]
c)
This question is about Step 3.
i)
Give the reagents and conditions needed.
[2]
ii)
Name the type of reaction taking place.
[1]
d)
State the molecular formula of benzyl propanoate.
[1]
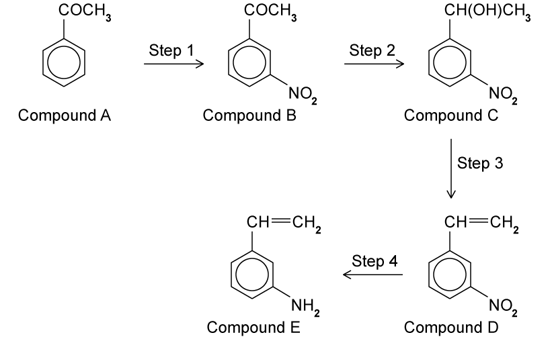
a)
The synthesis of 3-aminstyrene is shown below:
i)
Give the reagent needed in Step 1.
[1]
ii)
State the name of the functional groups in Compound B.
[2]
b)
This question is about Step 2.
i)
Give the reagent needed.
[1]
ii)
Name the type of reaction taking place.
[1]
c)
Step 3 is a dehydration reaction. Outline a chemical test that could distinguish between Compound C and the product of Step 3, Compound D.
[2]
d)
This question is about Step 4.
i)
State the name of the reagent(s) and conditions needed in Step 4.
[2]
ii)
Identify the type of reaction taking place.
[1]
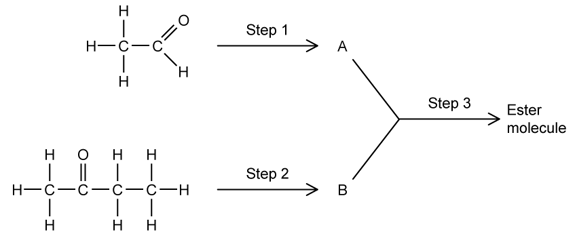
a)
This question is about the synthesis of an ester.
Identify the class of compound produced in Steps 1 and 2.
[2]
b)
This question is about Step 1.
i)
Give the reagent(s) and conditions needed to carry out Step 1.
[2]
ii)
Identify the type of reaction taking place.
[1]
c)
This question is about Step 2.
i)
Give the reagent(s) needed to carry out Step 2.
[1]
ii)
Identify the type of reaction taking place.
[1]
d)
Give the names of A and B and write the equation for the reaction in Step 3.
[3]
halogenoalkane Q
propan-1-ol