The reaction pathway for a reversible reaction is shown below: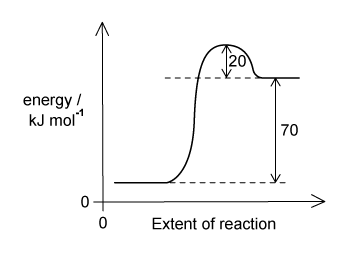
Which statement is correct?
The activation energy of the reverse reaction is +90 kJ mol–1
The activation energy of the forward reaction is +20 kJ mol–1
The activation of the reverse reaction is +20 kJ mol–1
The enthalpy change of forwards reaction is - 70 kJ mol–1
The reaction pathway for a reversible reaction is shown below.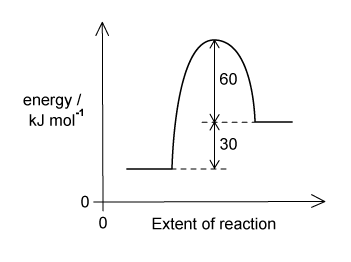
Which statements are correct?
|
I.
|
The forward reaction is endothermic
|
|
II.
|
The enthalpy change for the backward reaction is –30 kJ mol–1
|
|
III.
|
The activation energy for the forward reaction is +90 kJ mol–1
|
Hydrogen atoms bond covalently to iodine atoms to form hydrogen iodide as shown in the equation below:
H2(g) + I2(g) → 2HI(g)
Which statement best describes what is meant by the average HI bond enthalpy?
The energy stored in a covalent bond.
The energy required to break one covalent bond in the gas phase.
The energy required to break one mole of the HI bonds in the gas phase.
The energy released when two atoms form a covalent bond.
The enthalpy of atomisation of a compound can be calculated using a range of different enthalpy changes.
Which statement below correctly describes the enthalpy change of atomisation?
The energy stored in a covalent bond
The energy required to break one covalent bond in the gas phase
The energy required to break all bonds in an element
The energy released when two atoms form a covalent bond
Which quantity gives the best indication of the relative strength of the hydrogen bonds between water molecules in the liquid state?
Enthalpy changes of vaporisation
Bond dissociation energies
When a sample of calcium oxide, CaO, is added to dilute hydrochloric acid the temperature rises. Which of the following statements is correct?
More bonds are broken than are formed in the reaction
More bonds are formed than are broken in the reaction
The energy of the bonds broken is greater than of the bonds formed
The energy of the bonds broken is less than of the bonds formed
Which is the correct definition of mean bond enthalpy?
The amount of energy required to break a specific covalent bond in the gas phase
The energy required to break one mole of a specific covalent bond with all chemicals in their standard states
The amount of energy required to break a specific covalent bond with all chemicals in their standard states
The energy required to break one mole of a specific covalent bond in the gas phase, with all chemicals in their standard states
This energy profile diagram represents the reaction pathway for the following reaction:
Y (g) + Z (g) → W (g) + X (g)
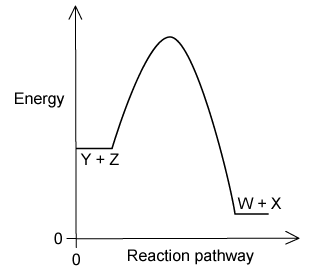
Which statement is true about the reverse reaction, W(g) + X(g) → Y(g) + Z(g)?
It will have a negative ΔH
It will have a positive ΔH
It will have a smaller activation energy
The temperature of the surroundings increase
Which of the following statements about oxygen and ozone are correct?
- Ozone contains delocalised π bonds
- The bond orders of oxygen and ozone are not the same
- The bond in oxygen requires radiation of higher energy and longer wavelength than the bond in ozone to break
The reaction of hydrogen with iodine to form hydrogen iodide is shown below:
H2 + I2 → 2HI
Use the bond energy data given to calculate the enthalpy of reaction, ΔHӨr.
|
Bond
|
Energy, kJ mol-1
|
|
H-H
|
432
|
|
I-I
|
149
|
|
H-I
|
295
|
ΔHӨr = 432 + 149 + (2 x 295)
ΔHӨr = 432 + 149 - (2 x 295)


