Question 1
Which definition of a Lewis acid is correct?
Electron pair donor
Electron pair acceptor
Proton donor
Proton acceptor
Which definition of a Lewis acid is correct?
Electron pair donor
Electron pair acceptor
Proton donor
Proton acceptor
Which type of titration is indicated by the following pH curve?
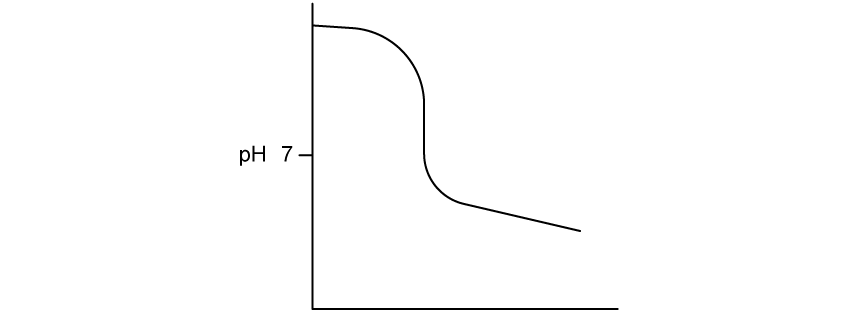
Strong acid - strong base
Weak acid - weak base
Strong acid - weak base
Weak acid - strong base
Which of the following best describes ammonia, NH3 , in the following equation?
BF3 + NH3 → BF3NH3
Lewis acid
Lewis base
Coordinate bond
Brønsted-Lowry acid
Which mixture cannot act as a buffer?
CH3COOH (aq) and CH3COONa (aq)
CH3CH2COOH (aq) and CH3CH2COONa (aq)
HNO3 (aq) and NaOH (aq)
NH3 (aq) and NH4Cl (aq)
The hydrolysis of ammonium chloride takes place via the following reaction.
NH4+ + H2O → H3O+ + NH3
Which of the following is correct?
The resultant pH is above 7
The resultant pH is 7
The resultant pH is below 7
Ammonium chloride is insoluble