Question 1
The structure of benzoic acid is shown below:
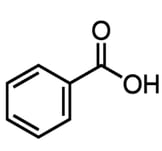
What is the correct number of sigma (σ) bonds and pi (π) electrons in benzoic acid?
| Number of sigma bonds | Number of pi electrons | |
| A. | 10 | 6 |
| B. | 15 | 8 |
| C. | 17 | 4 |
| D. | 9 | 10 |
The structure of benzoic acid is shown below:

What is the correct number of sigma (σ) bonds and pi (π) electrons in benzoic acid?
| Number of sigma bonds | Number of pi electrons | |
| A. | 10 | 6 |
| B. | 15 | 8 |
| C. | 17 | 4 |
| D. | 9 | 10 |
Which of the following correctly describes the F-S-F bond angles in sulfur tetrafluoride, SF4?
| Axial F-S-F bond angle / ° | Equatorial F-S-F bond angle / ° | |
| A. | 90 | 120 |
| B. | <90 | >120 |
| C. | <90 | <120 |
| D. | >90 | <120 |
Which of the following species is polar?
ICl4-
PF5
SO3
SH2
What are the formal charges on the atoms labelled 1, 2 and 3 on the hydrogen phosphate ion, HPO42-?
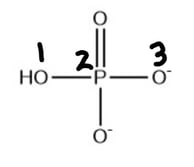
| Formal charge on atom 1 | Formal charge on atom 2 | Formal charge on atom 3 | |
| A. | 0 | 0 | -1 |
| B. | 0 | +1 | -1 |
| C. | -1 | 0 | -1 |
| D. | +1 | -2 | 0 |
The structure of the DNA base cytosine is shown below:
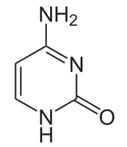
What is the correct number of sp3 and sp2 orbitals in a cytosine molecule?
| Number of sp3 orbitals | Number of sp2 orbitals | |
| A. | 2 | 6 |
| B. | 12 | 12 |
| C. | 9 | 15 |
| D. | 8 | 18 |