The rate information below was obtained for the following fourth order reaction at a constant temperature:
5Br– (aq) + BrO3– (aq) + 6H+ (aq) → 3Br2 (aq) + 3H2O (l)
Which rate expression is consistent with the data?
Rate = k [Br-] [BrO3-] [H+]
Rate = k [Br-] [BrO3-] [H+]2
Rate = k [Br-]2 [BrO3-] [H+]
The rate information below was obtained for the following reaction at a constant temperature:
C2H5Br (aq) + OH– (aq) → C2H5OH (aq) + Br– (aq)
|
[C2H5Br] / mol dm-3
|
[OH-] / mol dm-3
|
Rate / mol dm-3 s-1
|
|
2.0 x 10-3
|
1.0 x 10-2
|
4.0 x 10-4
|
|
4.0 x 10-3
|
1.0 x 10-2
|
8.0 x 10-4
|
|
8.0 x 10-3
|
2.0 x 10-2
|
3.2 x 10-3
|
What is the correct equation to calculate the value of the rate constant, k?
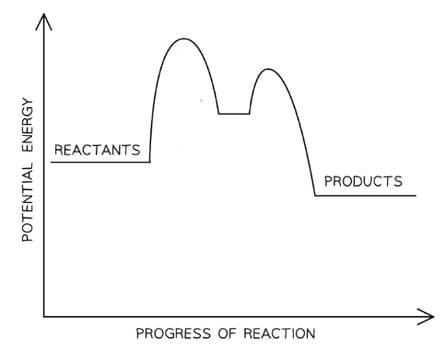
The mechanism for the following reaction between nitrogen(II) oxide and carbon monoxide is shown.
NO2 (g) + CO (g) → NO (g) + CO2 (g)
Step 1: NO2 + NO2 ⇌ N2O4 fast step
Step 2: N2O4 + 2CO → 2NO + 2CO2 slow step
Which rate expression is consistent with the mechanism?