Question 1
Which of the following calculations gives the correct calculation to find the energy, in kJ, for a photon of blue light given the wavelength ƛ = 550 nm.
h = 6.626 x 10−34J s; c = 2.988 x 108 m s-1
Which of the following calculations gives the correct calculation to find the energy, in kJ, for a photon of blue light given the wavelength ƛ = 550 nm.
h = 6.626 x 10−34J s; c = 2.988 x 108 m s-1
Successive ionisation energies for an element, Y, are shown in the table below.
| Electrons removed |
1st |
2nd |
3rd |
4th |
5th |
| Ionisation energy / kJ mol-1 |
736 |
1450 |
7740 |
10500 |
13600 |
What is the most likely formula for the ion of Y?
Y+
Y2+
Y3+
Y4+
Values for the successive ionisation energies for an unknown element are given in the table below.
|
First ionisation energy / kJ mol-1 |
Second ionisation energy / kJ mol-1 |
Third ionisation energy / kJ mol-1 |
Fourth ionisation energy / kJ mol-1 |
|
420 |
3600 |
4400 |
5900 |
In which group of the periodic table would the unknown element be found?
1
2
13
14
The graph shows the first ionisation energies of some consecutive elements
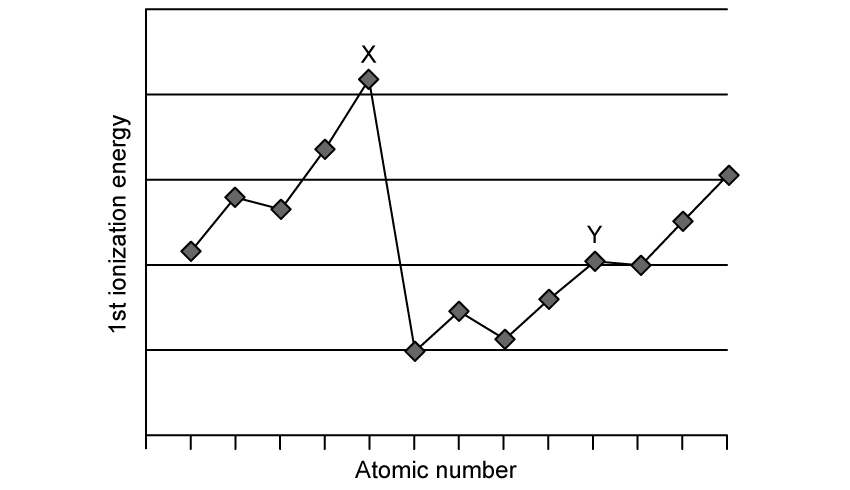 Which statement is correct?
Which statement is correct?
Y is in group 13
Y is in group 10
X is in group 15
X is in group 18
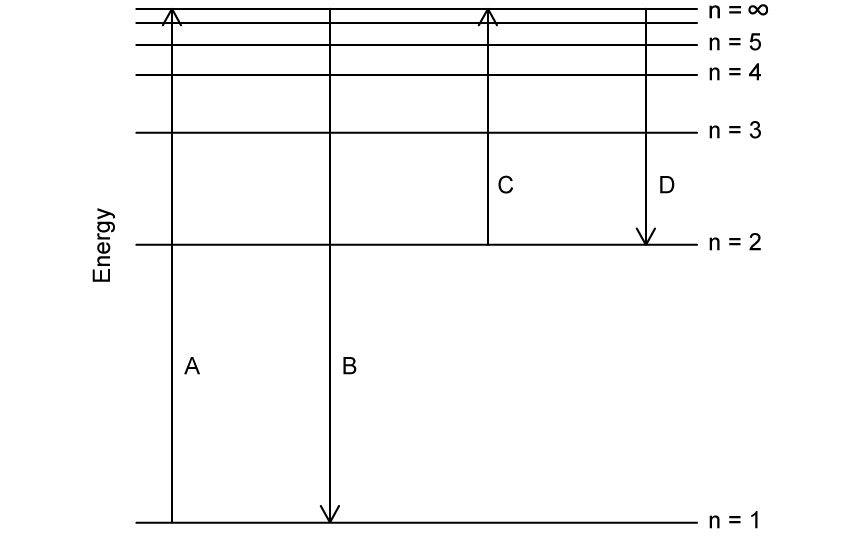
Which transition on the diagram corresponds to the ionisation of hydrogen in the ground state?