Which of the following statements are true for XeO3?
- Has a trigonal pyramidal shape
- One possible Lewis structure has two oxygen atoms with a formal charge of -1
- Xenon has a formal charge of +2 in one possible Lewis structure
Which species breaks the octet rule?
Which molecule is trigonal bipyramidal in shape?
How many sigma (σ) and pi (π) bonds are there in the following molecule?
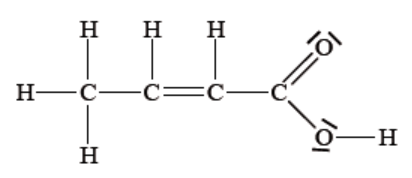
|
|
Sigma (σ) bonds
|
Pi (π) bonds
|
|
A
|
9
|
2
|
|
B
|
9
|
4
|
|
C
|
11
|
2
|
|
D
|
11
|
4
|
Which combination correctly describes the geometry of BrF4- ?
|
|
Electron domain geometry around Br
|
Molecular geometry around Br
|
|
A
|
Octahedral
|
Tetrahedral
|
|
B
|
Tetrahedral
|
Square planar
|
|
C
|
Octahedral
|
Square planar
|
|
D
|
Tetrahedral
|
Tetrahedral
|
