Question 1
Which compound contains delocalised electrons?
Cyclohexane, C6H12
Cyclohexene, C6H10
Benzene, C6H6
Cyclohexanol, C6H11OH
Which compound contains delocalised electrons?
Cyclohexane, C6H12
Cyclohexene, C6H10
Benzene, C6H6
Cyclohexanol, C6H11OH
Which structure does not show resonance?
Carbonate ion, CO32-
Hydroxide ion, OH-
Ethanoate ion, CH3COO-
Nitrate ion, NO3-
Which of the following cannot catalyse the decomposition of ozone, O3, to oxygen?
Cl
NO
O
CCl4
Which is the correct description of how an sp2 orbital is formed?
One s-orbital mixes with three p-orbitals
Two s-orbitals mixes with two p-orbitals
One s-orbital mixes with two p-orbitals
One s-orbital mixes with one p-orbital
The structure of glycine is shown below:
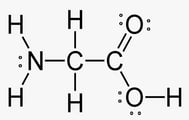
What is the correct number of sp2 and sp3 hybridized atoms in glycine?
| Number of sp2 hybridised atoms | Number of sp3 hybridised atoms | |
| A. | 8 | 2 |
| B. | 2 | 8 |
| C. | 3 | 2 |
| D. | 2 | 3 |