Question 1a
a)
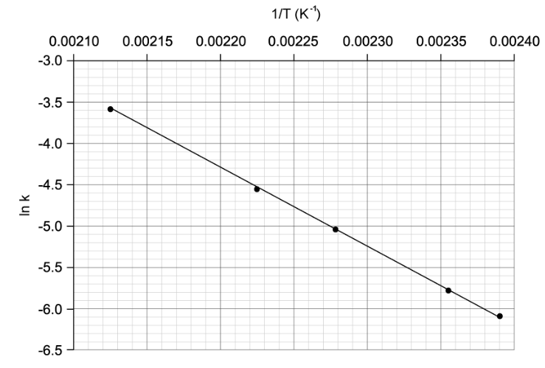
The Arrhenius equation can be written as:
State what each of the following terms represents, including units where applicable.
- A
- Ea
- R
- T
[5]
Question 1b
b)
Rearrange the Arrhenius equation given in part (a) to make A the subject.
[1]
Question 1c
c)
State how the rate constant, k varies with temperature, T.
[1]
Question 1d
d)
State how the activation energy, Ea, varies with rate constant, k.
[1]