Question 1
The graph below shows ln k against for a general reaction.
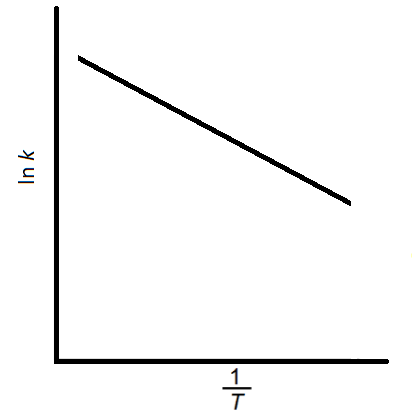
Which of the lines shows the highest activation energy compared to the original graph?
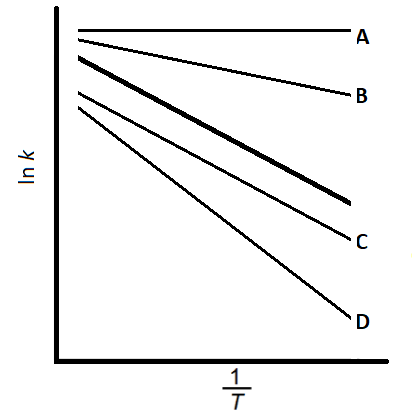
The graph below shows ln k against for a general reaction.
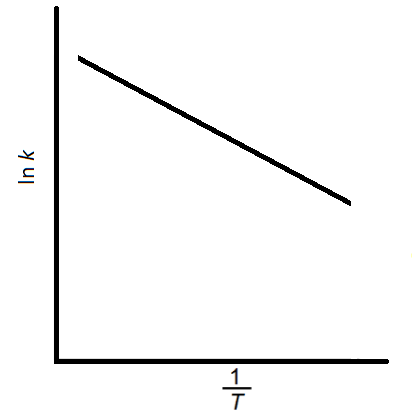
Which of the lines shows the highest activation energy compared to the original graph?

The following information was obtained for the rate constant, k, for a reaction at 298 K.
|
A |
Ea |
R |
|
2.57 × 109 s–1 |
96.2 kJ mol–1 |
8.31 J K–1 mol–1 |
Which expression correctly represents how to calculate the rate constant, k?
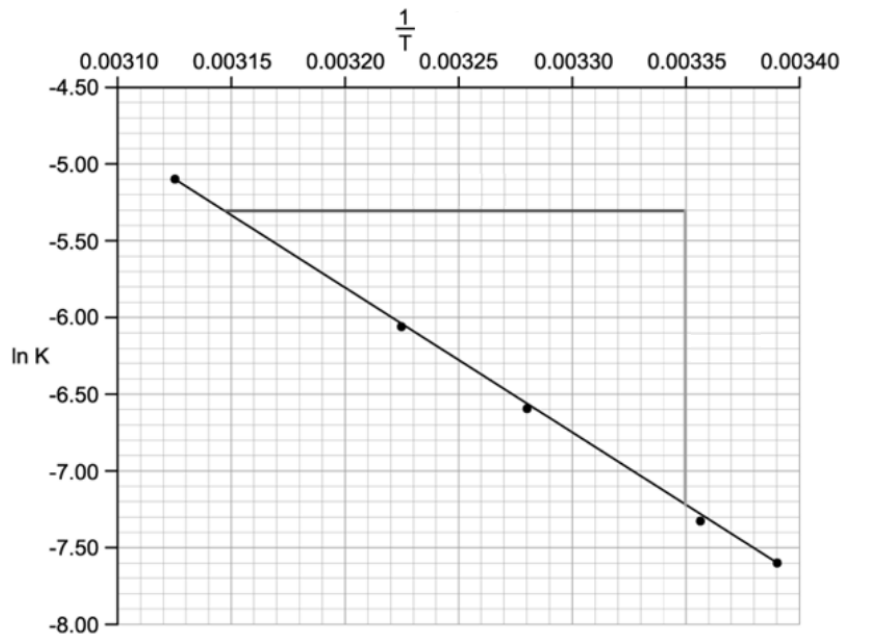
Which of the following statements about the Arrhenius plot are not correct?
ln A has an approximate value of -4.7
The gradient of the line is
The units for the x-axis are K-1
The equation of the line is ln k = + ln A
Which term from the Arrhenius equation has the incorrect units?
|
Term |
Units |
|
|
A. |
Ea |
J mol-1 |
|
B. |
R |
J K-1 mol-1 |
|
C. |
T |
K-1 |
|
D. |
e |
No units |
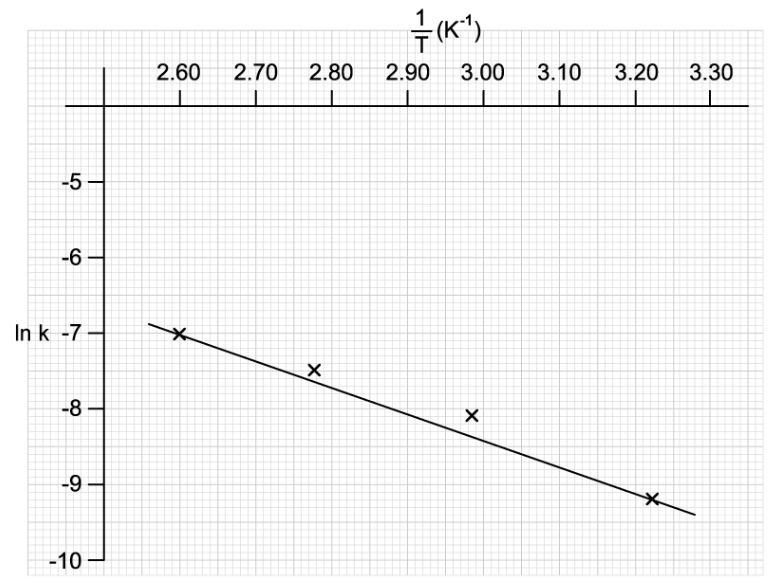
What is the gradient of the graph?
+ Ea
- Ea