Question 1
Which of the following can form a sigma (σ) bond?
I. Overlap between an s-orbital and a p-orbital
II. Overlap between two s-orbitals
III. Overlap between two p-orbitals
I only
I and II only
II and III only
I, II and III
Which of the following can form a sigma (σ) bond?
I. Overlap between an s-orbital and a p-orbital
II. Overlap between two s-orbitals
III. Overlap between two p-orbitals
I only
I and II only
II and III only
I, II and III
What is the correct number of sigma (σ) and pi (π) bonds in ethanoic anhydride, CH3COOCOCH3?
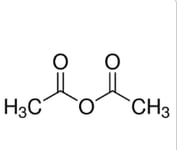
| Number of sigma (σ) bonds | Number of pi (π) bonds | |
| A. | 10 | 4 |
| B. | 10 | 2 |
| C. | 12 | 2 |
| D. | 12 | 4 |
Which element does not form stable compounds that break the octet rule?
Sulfur
Oxygen
Boron
Chlorine
What are the bond angles in a molecule with five electron domains, XY5?
| Axial bond angles / o | Equatorial bond angles / o | |
| A. | 90 | 90 |
| B. | 120 | 90 |
| C. | 90 | 120 |
| D. | 120 | 120 |
What is the correct formula to work out the formal charge on an atom?
FC = (Number of valence electrons) - (Number of bonding electrons) - (Number of non-bonding electrons)
FC = (Number of valence electrons) - (Number of bonding electrons) - (Number of non-bonding electrons)
FC = (Number of valence electrons) - (Number of non-bonding electrons) - (Number of bonding electrons)
FC = (Number of valence electrons) - (Number of non-bonding electrons) - (Number of bonding electrons)