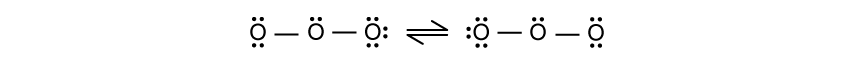a)
Benzene, C6 H6 , has two resonance structures. Draw skeletal formulae of these two structures.
[1]
Assess your score
View Answer
b)
Benzene is commonly drawn in the following manner:
Explain what this represents and why this is a useful way to draw benzene.
[2]
Assess your score
View Answer
c)
Some of the sigma bonds in benzene are formed from hybrid orbitals. The type of hybridisation present is sp2 .
State which orbitals hybridise to form sp2 orbitals.
[2]
Assess your score
View Answer
d)
The sp2 hybridized orbitals form sigma bonds in the benzene molecule. The delocalised electrons from pi bonds.
i)
Deduce the number of sigma (
) bonds in benzene.
[1]
ii)
Deduce the number of pi (
) bonds in benzene.
[1]
Assess your score
View Answer
Previous Question Next Question
a)
Ozone, O3 , forms two resonance structures, shown below:
i)
Allocate formal charges to the oxygen atoms in the left-hand diagram.
[1]
ii)
Deduce the bond order for the O-O bond in ozone.
[1]
Assess your score
View Answer
b)
The bond order of oxygen, O2 , molecules is 2.
i)
State which bonds are easier to break, those in oxygen or those in ozone.
[1]
ii)
Compare the wavelengths of light needed to break the bonds in oxygen and ozone respectively
[1]
Assess your score
View Answer
c)
Ozone, O3 , can react to form oxygen, O2 . Write an equation to show the overall equation for this depletion.
[1]
Assess your score
View Answer
d)
A number of species can catalyse the depletion of ozone, O3 .
Write the molecular formulae for two catalysts of ozone depletion.
[2]
Assess your score
View Answer
Previous Question Next Question
format('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22math1437d7d1d97917cd627a34a6a0f%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%226.5%22%20y%3D%2216%22%3E%26%23x3C0%3B%3C%2Ftext%3E%3C%2Fsvg%3E) electrons.
electrons.