a)
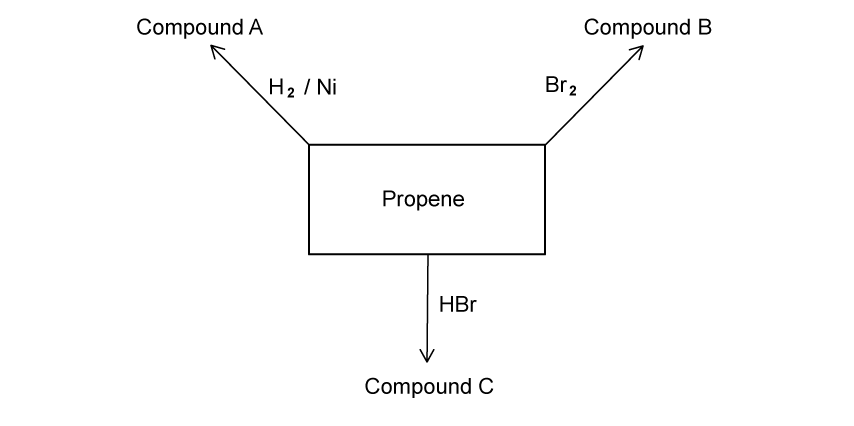
There are three steps to the free radical substitution mechanism. When ethane and chlorine react in the presence of UV light, chloroethane is produced. Write the equation for the initiation step.
Assess your score
View Answer
b)
Write two equations for the propagation steps for the reaction outlined in part (a).
Assess your score
View Answer
c)
Write the equation using structural formulae for the termination reaction between two CH 3 CH 2 ● free radicals.
Assess your score
View Answer
d)
State the type of bond breaking that occurs in the initiation reaction to produce free radicals.
Assess your score
View Answer
Previous Question Next Question
a)
State the balanced symbol equations for the complete combustion of propane and propanol.
Assess your score
View Answer
b)
The following reaction profile shown produces propanoic acid after three steps.
Step 1 Step 2 Step 3
Propene → Propan-1-ol → Propanal → Propanoic acid
State the reagents and conditions that can be used for steps 2 and 3.
Assess your score
View Answer
c)
Using your answer to part (b) to state the colour change for step 2.
Assess your score
View Answer
d)
Explain why 2-methylpropan-2-ol will not form a carboxylic acid.
Assess your score
View Answer
Previous Question Next Question
a)
Benzene undergoes substitution reactions. State the equation for the reaction of benzene with nitric acid to produce nitrobenzene and water.
Assess your score
View Answer
b)
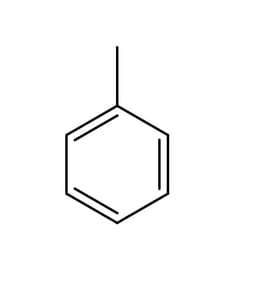
The structure of methylbenzene is shown below.
Draw the structures of the two isomers of choromethylbenzene formed from the reaction of methyl benzene and Cl2 in the presence of AlCl3 .
Assess your score
View Answer
c)
State the type of reaction that benzene will typically undergo.
Assess your score
View Answer
Previous Question Next Question