Photosynthetic pigments
 Students investigate a simple practical method of separating photosynthetic pigments (practical 4)using paper chromatography (or thin layer chromatography). Following this there is an animation of chromatography and some slides which outline how to calculate Rf values and identify pigments. Activity three outlines work for conclusions and evaluations. It includes some paper three style questions about the technique and the results analysis.
Students investigate a simple practical method of separating photosynthetic pigments (practical 4)using paper chromatography (or thin layer chromatography). Following this there is an animation of chromatography and some slides which outline how to calculate Rf values and identify pigments. Activity three outlines work for conclusions and evaluations. It includes some paper three style questions about the technique and the results analysis.
Lesson Description
Guiding Questions
Is the green colour of plant leaves due to a single pigment or more?Which of the following pigments is likely to move quickly in polar solvent (like water or alcohol)?
- Carotenoids (hydrophobic),
- Xanthophyll (hydrophilic),
- Chlorophyll A (hydrophobic)
- Chlorophyll B (hydrophobic)
Activity 1 - Separating photosynthetic pigments (Practical 4)
The following video shows the separation of pigments by chromatography speeded up ten times.
The method below explains how to carry out this experiment.
.
Experiment part 1 - Extract the Chlorophyll
Apparatus
- freshly picked leaves of Tradescantia pallidaa (purple in colour), or spinach (green, obviously!)
- propanone (acetone) (20ml per student)
- "Special Solvent" (90% petroleum ether + 10% propanone), (5ml per student)
- Dropping pipette
- Capillary tube
- pestle and mortar
- boiling tube with bung (or a beaker and watch glass)
- small quantity of washed and dried sand
- Kettle – to make a beaker of boiling water.
Safety
Consult the Student safety sheet on higher alcohols
- Wear safety glasses to avoid solvent contact with your eyes,
- No flames must be used during this lab - the solvents are very volatile & flammable!
Method
- Boil a kettle of water.
- Kill the cells of your leaf by holding them in boiling water for about 20 seconds.
- Cut out about 10g of leaf material, excluding the veins and stem using scissors.
- Crush the leaves with 20ml of pure propanone and a pinch of clean sand with a pestle and mortar.
Use this mix to take pigment for loading onto the chromatography paper.
Click the eye icon for an advanced separation method.
- Decant the solution using a glass rod to hold the leaf debris.
- Centrifuge the green solution to clear all the remaining suspended matter.
- Pour the supernatant into a small glass tube, but a bung in the tube and store in ice in the dark while you read the rest of the sheet.
Experiment part 2 - Separating pigments using Chromatography
Note: Only touch the edges of the chromatography paper.
- Cut a strip of chromatography paper to fit into your boiling tube without touching the sides
- Draw a pencil line across the paper, 2.5cm from the bottom end. This is the base line
- Put the "special solvent" (petroleum ether 90% & propanone 10%) into the tube up to 1.5cm depth.
- Replace the bung and allow the air in the tube to become saturated with solvent (about 10 mins).
- Put the strip of paper onto two glass rods (or pencils) so that the loading point on your base line is not touching the bench.
- Use a fine capillary tube to put a tiny spot of chlorophyll mixture onto the line on the paper, and allow to dry.
- Use a hair dryer to speed up drying.
- Add 10 more drops of pigment to the loading point.
- Put the paper back into the tube, avoiding letting it touch the edges of the tube, replace the bung
- Wait for the solvent to rise up the paper to 20cm from the bung. (keep looking)
- Remove the paper and mark the solvent front with a pencil line.
- Hang up to dry (in a dark place if possible.)
- When dry, mark the centre of each individual pigment spot on the paper and record its colour,
Activity 2 - Simulation of chromatography & identification of pigments
This animation shows how the pigments in a sample of leaf extract separate during chromatography.
The practical details are described in Activity 1 above.
Chromatography questions
1. Some pigments stick to the paper more than others, will they travel more quickly or more slowly?
.............................................................................................
2. Other pigments dissolve in the solvent more easily, will the travel up the paper more quickly, or more slowly?
.............................................................................................
3. Why do the pigments move at different speeds?
.............................................................................................
4. Once separated, how can these photosynthetic pigments be identified?
.............................................................................................
Chromatography questions - model answers
1. Some pigments stick to the paper more than others, will they travel more quickly or more slowly?
...........More slowly because sticking to the paper stops the molecules moving............
2. Other pigments dissolve in the solvent more easily, will they travel up the paper more quickly, or more slowly?
............More quickly because they detach from the paper more and travel with the solvent more ............
3. Why do the pigments move at different speeds?
........... Different pigments have different chemical properties which causes the different speeds
relating to the properties or adhesion to the paper, or dissolving in the solvent. ........
4. Once separated, how can these photosynthetic pigments be identified?
............. The colour and the Rf values of the pigments can be used to identify them ..........
Study the slides below which illustrate the calculation of Rf values.
These values are specific to each pigment but they also depend on the type of paper and the solvent being used.
Carry out the practice calculation of Rf values on the ![]() Photosynthetic pigment identification worksheet.
Photosynthetic pigment identification worksheet.
Activity 3 - Conclusion and Evaluation
This is an image taken from the wet lab which could be used in the conclusion below if required.
The two samples A & B are from different species of plants
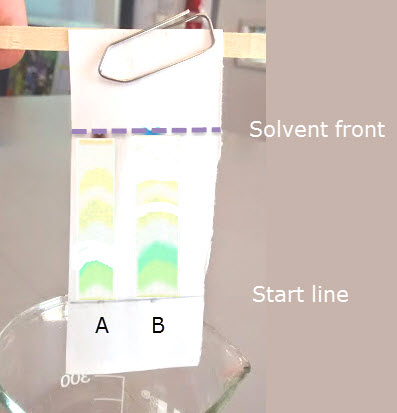
Conclusion
- Describe the colours of the pigments you have found
- Calculate the Rf value for each pigment
- Using the information in the Rf table above, work out which pigment is in each band.
- Compare what you find to a text book which gives details of the photosynthetic pigments found in leaves.
Background theory about solvents
Photosynthetic pigments are present in the chloroplasts of plant cells and absorb light. There are a number of pigments which absorb different wavelengths of light. Green light is mostly reflected. The pigments are attached to membranes in the chloroplast called thylakoids and grana.
Some pigments are hydrophilic (propanone) and others hydrophobic (petroleum ether).
Hydrophilic molecules are polar and have electrical charges (e.g. phosphate of phospholipid). They are soluble in water and propanone and insoluble in petroleum ether, lipid, oils.
Hydrophobic molecules are non-polar and have no electrically charged areas. (e.g. fatty acid chains in phospholipids). They are insoluble in water and propanone. Soluble in petroleum ether, oils and lipids.
We can separate them in chromoatography using a mix of two solvents one hydrophilic and the other hydrophobic. A little water is also needed to help mix them but this is usually found in the propanone already. . For these we will use "Petroleum ether" and "Propanone". The hydrophilic pigments will dissolve in the hydrophilic solvent and vice versa.
Evaluation
- Describe the limitations of the method.
- Explain the significance of this limitation.
- Suggest a way to improve each of the limitations you have identified.
- Complete the table below if you prefer this format.
|
Limitation of the experiment |
How did this effect the results |
How to eliminate this limitation in a future experiment |
Teachers' notes
This learning activity could take the form of a wet lab and might take a couple of hours to carry out and write up.
Note that the Rf values may be different in different solvents or with different types of paper so some discrepancy is possible.
In paper 3 their could be questions to calculate Rf values, compare different results from different species or simple discussions about the limitations of the techniques. It is advisable to carry out this experiment in some form or other, even if the results produced don't give enough data for a detailed analysis.
The data provided in this learning activity could be used for analysis in such cases.
There are some extra, interesting experiments on this sheet from the University of Miami. They could help to inspire a student looking for an idea for an individual investigation.
Solvents
Sometimes the choice of solvent and the choice of leaf can make a difference to the success of this experiment.
In the literature there are a range of solvents used. Essentially the solvent should be a mixture of a polar solvent and a non polar solvent. Ether is non-polar, acetone is slightly polar, and water is of course very polar. I have seen the following mixtures used for chromatography of photosynthetic pigments:
- 80% petroleum ether with 20% acetone,
- 80% acetone and 20% pet. ether,
- 60% acetone with 20% Pet ether and 20% water
- 100% acetone.
- Running a coin across a softened spinach leaf several times without a solvent

 IB Docs (2) Team
IB Docs (2) Team





