Visualising molecules
Identification of biochemicals.

Using online flashcards and a database, students learn how to identify biological molecules and to distinguish between them. Examples included are monosaccharides such as alpha-D-glucose, beta-D-glucose and D-ribose, a disaccharide and lipids such as a saturated fatty acid, a triglyceride, and a phospholipid. There is also a polypeptide, two amino acids linked by a peptide bond and a generalized amino acid.
Lesson Description
Guiding Questions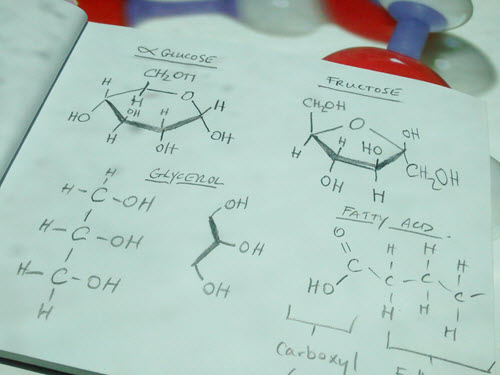
Can you recognise the structures of some important biological molecules, like glucose and fructose?
How might the size and shape of the molecules change their properties?
How is a starch molecule different to a hundred glucose molecules?
Activity 1 - Key points about biological molecules
Using these online flashcards practise some of the details about the structure of these biological macromolecules. (click the eye to show the flashcards)
Activity 2 - Visualising molecules
Answer the questions below using the 3D visualisations of biological molecules. Pause the video to see the molecule or click the link to the Jmol database and manipulate the molecules directly on Chemspider.
There is also a ![]() Visualising molecules student worksheet.
Visualising molecules student worksheet.
In the images carbon is coloured grey, oxygen is red and hydrogen white. In proteins nitrogen is blue.
Monosaccharides
- What is the difference between alpha and beta glucose?
- How does D-ribose differ from the two glucose molecules.
alpha-D-glucose |
beta-D-glucose |
D-ribose |
Disaccharides
- What is the characteristic feature of these three disaccharides?
- How many carbon atoms do each of the monosaccharide components of the disaccharides have?
Maltose |
Sucrose |
Lactose |
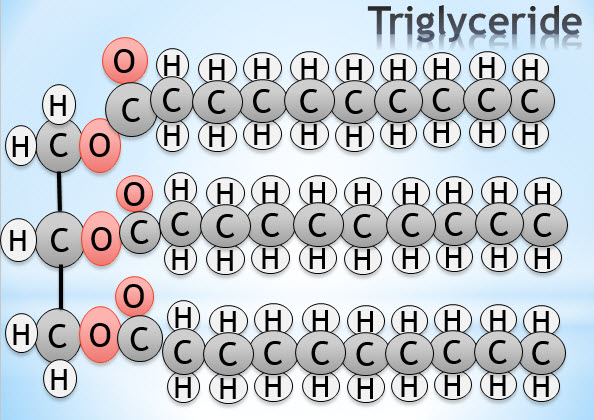
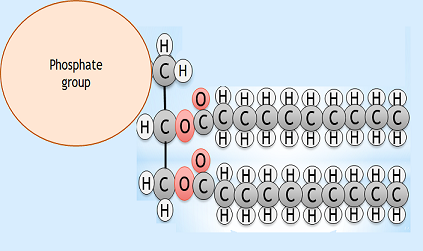
Lipids
- What are the two main differences between a phospholipid and a triglyceride?
- How does a saturated fatty acid attach to glycerol in a triglyceride?
- Which of these molecules most resembles cholesterol? Why?
Saturated fatty acid |
Triglyceride diagram
|
Phospholipid diagram
|
Glycerol |
A Steroid - Cholesterol |
Another Triglyceride |
Proteins
- Describe the two ends of the amino acid Glycine - this is the simplest amino acid as the R-group is just Hydrogen.
- Which two atoms bond together to form a peptide bond between two amino acids?
- How can you spot a peptide bond in larger molecules?
- In a polypeptide what are the molecular groups at each end of the chain?
Glycine
|
Two Amino acids (linked by a peptide bond) |
Polypeptide (with 4 amino acids) |
Further details about the structures of some of these molecules can be found by clicking the links to Chemspider by each video above. Chemspider is a free chemical structure database providing structures and chemical properties of over 32 million structures.
Student worksheet of questions
Answer the following questions as you make notes: ![]() Visualising molecules worksheet
Visualising molecules worksheet
Activity 3 IB Style Questions
Test your knowledge with these ![]() IB style questions about biological molecules which you have just studied.
IB style questions about biological molecules which you have just studied.
Teachers notes
NoS TOK - Opportunity
This activity could be used to illustrate some points about NOS and TOK and engage the students in a really interesting analysis of the usefulness in Biology of 2D diagrams of 3D chemicals.
Some scientists draw Ribose with the -OH and -H groups in different positions in a 2D diagram.
Are they wrong? How is it possible that there can be disagreement?
Take a look at this image of Ribose it has the -OH group on C1 carbon going upwards. In IB past paper question in May 2004 and 2008 there were molecules of ribose with the C1 -OH going down. I couldn't find a diagram the other way round.
Which diagram is wrong? Could they both be right?
The problem comes from trying to represent a 3D molecule in a simplified 2D diagram.
In fact the four bonds on a Carbon atom (in methane) form a tetrahedral shape. The diagrams we learned to draw last lesson are simplifications. I would argue that they are useful 2D models because they help biology students to understand the nature of biological molecules and from that understanding the functioning of enzymes and the properties of cell membranes and other cell organelles. All of this without worrying about 3D shape and bond angles - which IB chemists have to do.
In RIbose if the -O- is at the top and C1 on the right then the C1 -OH group comes forwards. See this video clip.
If you view from slightly above then -OH looks lower than H on the C1. If you look from below then the -OH looks higher.
The best way to see is using the 3D rendering of the Chemspider 3D JS model seen in this lesson.
I think this is a really interesting point for discussion with students. It would cover the NOS points on the use of models (1.10) and it would address the TOK dilemma between simplification to aid understanding and the the accuracy of complex models.
This problem of experts disagreeing features in TOK Essay titles:
Title 5 (2007):
Given access to the same facts, how is it possible that there can be disagreement between experts in a discipline?
In fact, like many monosaccharides, ribose exists in an equilibrium among 5 forms:
- the linear form
- alpha-ribofuranose
- beta-ribofuranose
- alpha-ribopyranose
- beta-ribopyranose.
The beta-ribopyranose form predominates in aqueous solution (59%).
The ribose form β-D-ribofuranose(13%) forms part of the backbone of RNA.

The aim of this lesson is to "flip the classroom" so that students make the best use of valuable lab time to complete the experiment while covering the factual theory work as homework, preferably before the lesson.
The IB-style questions can be used to check that the students have understood the simple facts and molecular names.
The Jmol 3D visualisations of molecules is a great introduction to Jmol and the possibilities of this sort of ICT. This is a simple biological gallery of Jmol 3D molecule visualisations.
The model answers are on separate pages so that teachers can control student access to these pags.
- This link model answers for this visualising molecules page for model answers to activity 2
- This link Bio molecules IB style model answers will take you to some model answers for the IB style questions in activity 3.

 IB Docs (2) Team
IB Docs (2) Team