Drawing biological molecules
 Students learn how to draw the following molecules; glucose, ribose, saturated fatty acid and an amino acid. They answers questions which gives practice in recognition of each molecule activity. This is followed by an extension reading on vitalism and urea.
Students learn how to draw the following molecules; glucose, ribose, saturated fatty acid and an amino acid. They answers questions which gives practice in recognition of each molecule activity. This is followed by an extension reading on vitalism and urea.
Lesson Description
.jpg) Guiding Questions
Guiding Questions
Are living organisms more than the sum of all the chemicals which make them?
Do biological chemicals contain a vital force?
Activity 1 - Drawing molecular diagrams
When drawing molecular diagrams which show all the atoms which make up a molecule and the way in which they are bonded together it is first important to know how many bonds each molecule has.

When drawing the biological molecules checking that each atom has the right number of bonds, will avoid mistakes.
A good way to remember the structures is to draw each diagram in three steps, the central atoms, the functional groups and then the hydrogen atoms.
Carbohydrates:
- Draw the hexagonal, or pentagon shaped 'ring' of carbon atoms (C)
- Add the oxygen (O) to complete the 'ring'.
- Put hydroxyl groups (OH) in the appropriate position on each carbon
- Fill in all the remaining bonds with hydrogen (H) using the number of bonds above.
Fatty acids:
- Draw the central carbon atoms ( -C-C-C- etc)
- Add -OH and =O to one of the end C atoms to make it a carboxyl group.
- Fill in all the remaining bonds with hydrogen (H) using the number of bonds above.
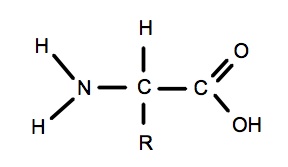
Amino acids:
- Draw the central N-C-C atoms
- Add the R-group to the central C
- Add -OH and =O to the end C to make it a carboxyl group.
- Fill in all the remaining bonds with hydrogen (H) remembering that N only has three bonds total.
Drawing α-glucose (alpha-glucose)
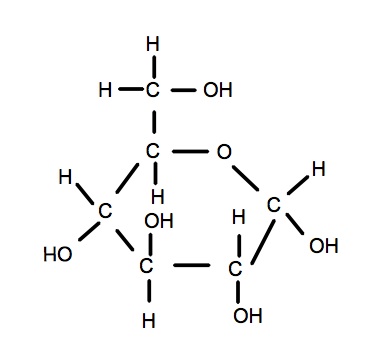 |
Show hints:
- Draw the hexagon using 5Cs and 1O
- Add the 6th C above the 5th C
- Draw the -OH groups in their correct positions
(this is the difference between alpha and beta glucose) - It is correct to draw -OH or HO- ( but not OH- or -HO.)
- Add H2OH to the 6th C
- Add -H to all the carbons to make 4 bonds per carbon
Drawing β-glucose (beta-glucose)
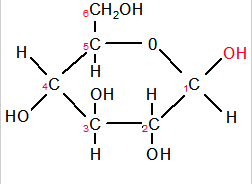 |
Show hints:
- Make a note of the similarities between beta-glucose and alpha-glucose
- Beta glucose has all the same parts as its alpha glucose isomer
- Simply take care to draw the -OH groups in their correct positions
(for beta glucose the OH on the 1st C must be at the top)
Drawing ribose
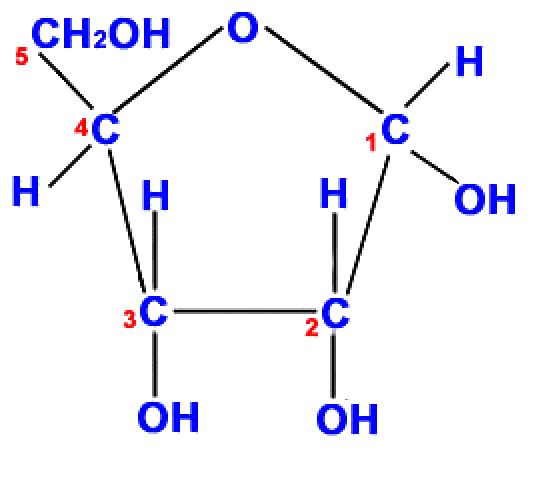 |
Show hints:
- Ribose is similar to glucose in several ways:
- carbons 1, 2 & 3 all have an H and an OH attached.
- the last carbon(5) is also CH2OH.
- Ribose only has 5 carbon atoms
- Draw a pentagon using 4 Cs and with an O at the top
- Add a 5th carbon above the 4th C.
- Add H2OH to the 5th carbon
- Add 3 OH groups beneath Carbons 1, 2 & 3.
- Add a H to all the carbon atoms with fewer than 4 bonds.
Drawing a saturated fatty acid
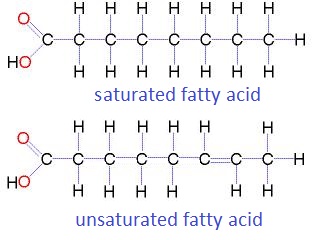 |
Show hints:
- Draw 8 carbon atoms in a chain
- Add the carboxly group =O and -OH to the first carbon
- Add hydrogen atoms to all the carbons to make 4 bonds per carbon atom
- note the last carbon needs three H atoms.
Drawing an amino acid
 |
Show hints:
- This is similar to a fatty acid because of the carboxyl group (C=O & -OH)
- Remember N has just 3 bonds
- The R group represents the area of the molecule which is different in each amino acid.
Student worksheet
Students can complete a copy of the diagrams on ![]() Student worksheet - drawing molecules
Student worksheet - drawing molecules
Activity 2 - The slaying of a beautiful hypothesis by an ugly fact
Read one or more of the following articles and make notes to explain how the synthesis of urea helped to disprove the theory of vitalism - the idea that there is a 'vital force' in living organisms as well as the biochemicals.
This article, kindly suggested by Troy Glover, is an excellent detailed account of the theory of vitalism:
Teachers notes
This lesson could be a homework activity or a self study lesson.
Each of the molecules is shown in two diagrams both deliberately slightly different so that students realise that there is more than one way to represent the molecules. The carbon atoms have all been represented as C and the simplified stick diagrams have not been used.
This is lesson is not inquiry based but the hints with each drawing help students to make links between molecules and to find similar structures. Learning how to draw these molecules will involve some practice.
Pictionary or Articulate revision games
A fun way to practice drawing these diagrams might be to ask students to work in pairs and play a game of pictionary or articulate using the diagrams.
Pictionary. One student draws structures while the other guesses their names for a limited time, e.g. 1 minute. The winners are the team which guess most correct in the time.
Articulate. The students work again in pairs and take turns to describe the structures in words, without using the chemical names just the positions of the atoms in relation to one another. The winning pair are the ones who correctly identify the most molecules in 1 minute.

 IB Docs (2) Team
IB Docs (2) Team
