Toxicology training course
Emergency room training activity - enzyme inhibition
Read the following medical briefing sheets - imagine that you are a junior doctor doing a toxicology course.
Answer the questions at the end of each section and keep brief notes for yourself.
Alcohol metabolism, enyzyme inhibition & toxicology
 .
.
.
Course contents
- Introduction to alcohol metabolism
- Symptoms of alcohol intoxication
- Ethylene glycol metabolism
- Symptoms of intoxication
- Treatment option 1 - using a competitive inhibitors of an enzyme
- Treatment option 2 - using a non-competitive inhibitor
- Treatment option 3 - dialysis
.
.
After each section there is a self-review question. Answer this question correctly before continuing.
Test yourself now to see if you are ready to begin this course.
Question 1
Which is the best description of a cell's metabolism?
If you answered correctly, Well done. If not, review the key information you didn't know.
Introduction to alcohol metabolism & Symptoms of alcohol intoxication
The metabolic pathway of alcohol metabolism begins in the liver with the enzyme, "alcohol dehydrogenase" this enzyme produces acetaldehyde which is the substrate for the second enzyme in the pathway, "acetaldehyde dehydrogenase".

The alcohol leads to symptoms such as
- Reduced inhibition in social situations
- Excessive talking, showing off
- Ataxia (reduced coordination)
- Loss of memory
- Slurred speech
- Vomiting
The acetaldehyde produced is largely responsible for the hangover symptoms which occur 12-24 hours after alcohol intoxication.
Both these dehydrogenase enzymes remove hydrogen from their substrates which is carried by reduced NAD (NADH + H+)
This acetic acid produced by acetaldehyde dehydrogenase is converted into acetyl-co A which enters the krebs cycle in mitochondria of the liver.
Some alcohol is excreted by the lungs (5%) or the kidneys (5%) and can be tested for in breath of in the urine.
Question 2
What is the name of the first enzyme in alcohol metabolism?
If you answered correctly, Well done. If not, please review the key information you didn't know before continuing.
Ethylene glycol metabolism & symptoms of intoxication
Ethylene glycol is a clear, colorless, odorless, sweet-tasting liquid.
Ethylene glycol, HOCH2CH2OH has a chemical structures similar to ethanol, CH3CH2OH
Ethylene glycol is rapidly absorbed from the intestines within 30-60 minutes following ingestion. It is water soluble.
The toxicity of ethylene glycol results from its metabolism, catalysed by enzymes in the liver, which produce more toxic chemicals.
This metabolic pathway breaks down ethylene glycol quickly in the body (in a few hours) into glycoaldehyde, then into acids which change blood pH and oxalic acid which reacts with calcium to form calcium oxalate crystals which damage heart, lung and blood vessel tissues.

Symptoms or ethylene-glycol intoxication are at first similar to alcholol intoxication.
- ataxia (reduced coordination)
- sleepiness
- slurred speech
Once glycoaldehyde begins to form the CNS can be severly depressed leading to
- siezure - where electrical activity in the brain changes
- vomiting
Symptoms of the later metabolic products are often;
- fluid on the lungs or lung inflamation leading to rapid breathing (hyperventilation)
- heart problems leading to rapid heart rate
- problems in blood vessels leading to changes in blood pressure (higher or lower)
In serious intoxication the symptoms above are often fatal, but if not, calcium oxalate crystals form in the kidney tubules at later stages causing kidney failure.
Question 3
Why is ethylene glycol so much more dangerous than alcohol?
If you answered correctly, Well done. If not, please review the key information you didn't know before continuing.
Treatment option 1 - using Ethanol as a competitive inhibitors of an enzyme
The most important way to prevent poisoning by the metabolic products of ethylene glycol is to stop their production.
 To do this we need to inhibit the action of the enzyme alcohol dehydrogenase. This is the enzyme which starts the metabolism.
To do this we need to inhibit the action of the enzyme alcohol dehydrogenase. This is the enzyme which starts the metabolism.
Remember that the ethylene glycol first has to bind to the active site of the enzyme. Hydrogen is removed and the products are released.
One way to stop the ethylene glycol from binding to the active site is to fill the active site with a different molecule which also fits into the active site .... like ethanol.
Ethanol is a competitive inhibitor in this case - it competes with the substrate (ethylene glycol) for the active site of the enzyme
Ethanol reacts in the same way, but doesn't produce the same toxic metabolic 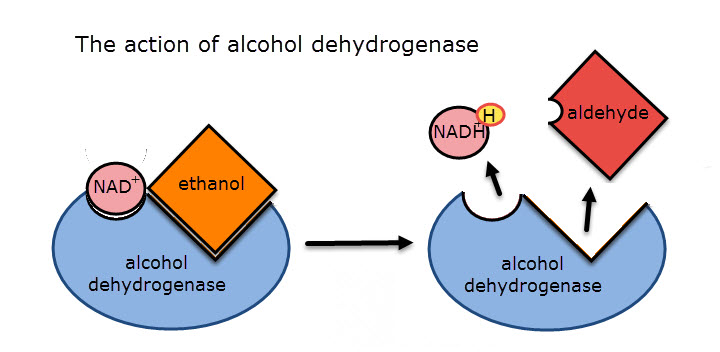 products.
products.
One treatment option is to keep the patients blood ethanol level raised for three days. This slows down the breakdown of ethylene glycol which gives more time for the body to excrete the unchanged ethylene glycol by the kidneys.
Of course there are problems because the ethanol level needs monitoring, and the patient will be 'drunk' for three days.
.
Competitive inhibition reduces a reaction rate by preventing the substrate from binding to the active site.
The higher the concentration the more inhibition there is.
Question 4
Which is the best definition of a competitive inhibitor?
If you answered correctly, Well done. If not, please review the key information you didn't know before continuing.
Treatment option 2 - a new competitive inhibitor "Fomezipole"
For detailed information about this medicine see the Drugs.com database
This medicine acts as a competitive inhibotor of the alcohol dehydrogenase enzyme and slows the production of the toxic metabolic products, just like ethanol.
It has several advantages:
- is easier to use clinically and requires less monitoring
- has a slower rate of elimination
- has a longer duration of action
- has a reasonable dosing schedule
- has less potential for adverse effects
- does not cause CNS depression or hypoglycemia
- avoids problems in patient care, such as drunkenness
Disadvantages
One disadvantage is that fomepizole is more expensive than ethanol treatment.
.
Question 6
What is the main similarity between Fomepizole and ethanol?
If you answered correctly, Well done. If not, please review the key information you didn't know before continuing.
Treatment option 3 - dialysis
If the concentration of ethylene glycol is very high in the blood it is essential to remove some of this as quickly as possible. To do this the patient can have a treatment of dialysis. The ethylene glycol will diffuse across the dialysis membrane.
Question 7
Why is it impossible to prevent the alcohol dehydrogenase enzymes from breaking down the ethylene glycol using competitive inhibitors when there is a high concentration of ethylene glycol in the body?
With a high concentration of ethylene glycol the alcohol dehydrogenase will be working too quickly
Well done. You have completed this short training course. You are now ready to try some case studies.

 IB Docs (2) Team
IB Docs (2) Team

