Properties of Water
Hydrogen bonding, the properties of water and its use in living things.
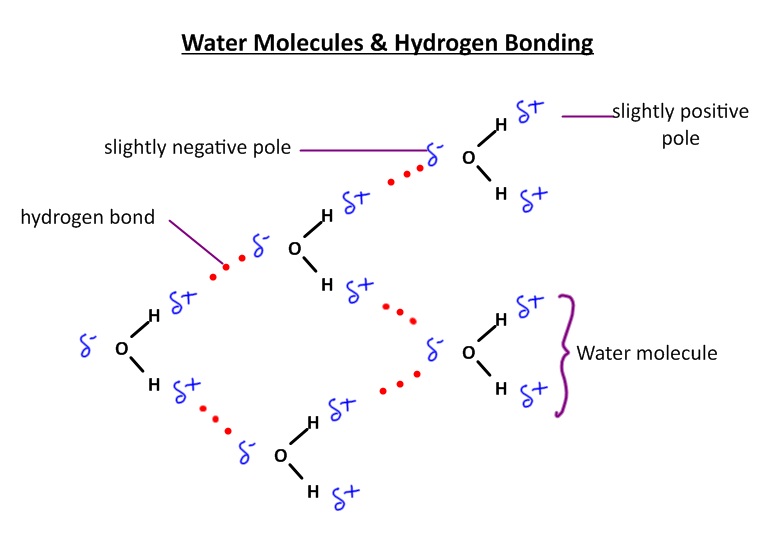 This lesson uses a screen cast to introduce a simple diagram of hydrogen bonding in water. There are a range of activities to help student to understand how these hydrogen bonds affect the properties of water and how this affects living things. This includes activities to compare water and methane as well as solubility of organic molecules in water using the possibility of extra terrestrial live as a context.
This lesson uses a screen cast to introduce a simple diagram of hydrogen bonding in water. There are a range of activities to help student to understand how these hydrogen bonds affect the properties of water and how this affects living things. This includes activities to compare water and methane as well as solubility of organic molecules in water using the possibility of extra terrestrial live as a context.
Lesson Description
Guiding Question
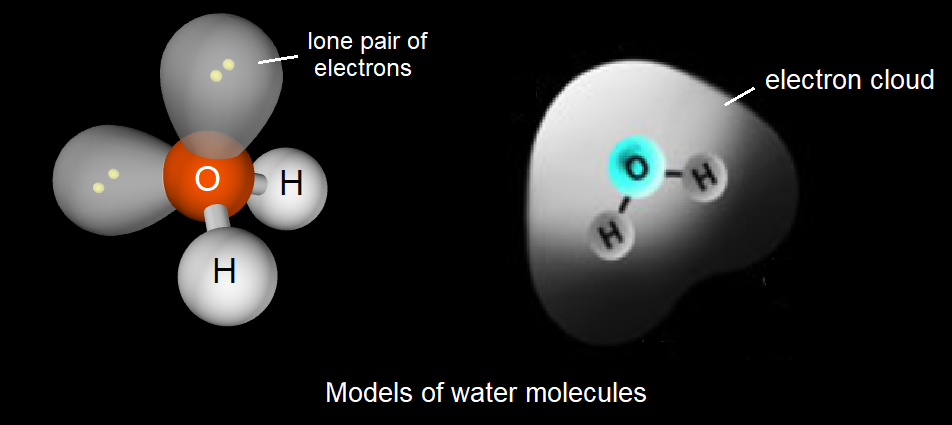
In an atom or molecule the electrons are not fixed in place but they move rapidly, occupying a 'cloud' of space.
In these images what evidence is there that electrons may not be evenly distributed in water molecules?
Activity 1 - Draw a diagram of polar water molecules
Play this short video ![]() Drawing a diagram of polar water molecules and use it to complete the
Drawing a diagram of polar water molecules and use it to complete the ![]() water diagram activity sheet (shown below).
water diagram activity sheet (shown below).
Activity 2 - Learn some of the properties of water
Use ![]() the properties of water flashcards below to familiarise yourself with some of the properties of water.
the properties of water flashcards below to familiarise yourself with some of the properties of water.
Quick Test
Study the flashcards on Quizlet
Quick test - Online (self marking)
Try the questions below to test your understanding.
It is possible to change the question types and personalise the challenge using the options button.
Activity 3 - Comparing the properties of water and methane
Saturn's moon Titan has lakes containing methane, it rains methane and it's water is as hard as rock. So what is the difference between water and methane?
Watch the short video clip about Titan's atmosphere. (40s)
Questions to answer
on the ![]() Extra-terrestrial life on Titan worksheet
Extra-terrestrial life on Titan worksheet
- What state is methane, solid liquid or gas, in the earth's atmosphere?
- Why is it that methane can evaporate at -180°C on Titan whereas water is frozen solid at this temperature?
- What is the "special force" which is found between water molecules that is not found in methane?
- What effect does this force have on the melting point and boiling point of water?
- What might this do to the cohesion of water molecules compared to methane molecules?
- Sweating cools the skin because the water in sweat evaporates and carries heat away from the skin. If humans made sweat with methane instead of water how would it change the effectiveness of sweating to cool the skin?
- If extraterrestrial life on Titan does exist, and if it uses methane in sweat to cool down, explain why Titan's organisms would be happy to be sweating with methane and not water.
- Another difference between Titans lakes and the oceans of the earth will be the substances dissolved in each. Water is a polar molecule and an excellent solvent of ionic substances like salts and other polar molecules like amino acids and sugars. Methane is non-polar and thus will dissolve lipids and small molecules better. Suggest why cytoplasm or blood made with water would be better for living things than cytoplasm or blood made from methane.
Extension Reading
Click the eye icon to open a list of further reading
Teachers' notes
This page is part of a series of lesson resources for the diagrams of the IB syllabus.
The simple diagram is designed especially as one which is easy for students to replicate under exam conditions. A more detailed diagram link is always given to support understanding and raise awareness of the beauty and complexity of biological systems. There are some alternative explanations here:
- Flash slide show - explains water molecules & polarity nicely,
 A good animation of hydrogen bonding in water
A good animation of hydrogen bonding in water
There are many ways to use this page.
- Students can work independently or as a group.
- The resources can be projected in the classroom or studied on a computer.
- Worksheets allow you to print as much or as little as you wish.
- Differentiation is possible, as students could follow their own learning pathways to the IB style questions.
There are model answers for activity 3 on this page: Extra terrestrial life - model answers
A Suggested Lesson Plan.
Starter: 10 minutes
- Students complete the Diagram Activity worksheet while watching the Screen cast individually of as a whole class.
Main: 40 minutes
- Students study printed (or online) Flashcards to help with properties of water.
- Students take a Quick test - to see how well they understand the properties.
- Students watch the short video and answer the questions on water compared to methane.
End: 10 minutes
- Plenary - review the properties of water and the role of hydrogen bonding.
Homework
Could be to write an essay about the properties of water and how they are useful for life on earth.
In addition
There is also a nice set of resources for a nice circus of experiments on water properties here
Simple circus of water properties experiments - from Penncrest High School
A TED video about Titan Saturn's largest moon - explains a new NASA mission of 2026.
What Saturn's most mysterious moon could teach us about the origins of life | Elizabeth Turtle
There are some good chemical activities about methane and water here:
Water_vs_Methane.pdf (uploaded by Cat Carrington)

 IB Docs (2) Team
IB Docs (2) Team
