Calorimetry experiment
Properties of carbohydrate and lipid molecules and calorimetry.

A great simple experiment to illustrate the energy content of these two organic molecules used for energy storage. Students carry out a calorific test on lipids and carbohydrates. They use visualisations from jmol of the structure of cellulose and starch (amylose & amylopectin) and glycogen to answer questions about the experiment's results and how each molecule relates to its function.
Lesson Description
 Guiding Questions
Guiding Questions
How many different types or molecule could be made by linking simple sugars like glucose and fructose together?
How might these different structures affect their properties?
Activity 1 - Experiment to test the energy content of Carbohydrate & Lipid
A great simple experiment to illustrate the energy content of these two organic molecules used for energy storage.
The experiment as described will show that potato cooked in fat will contain more energy than dried potato (rich in carbohydrates) alone. Alternatives to the experiment can include using low fat crisps, or even carefully burning sugars, and oils in a spoon.
The mass of each sample should be measured so that a meaningful comparison can be made.
![]() Calorimetry experiment - the energy value of potatoes
Calorimetry experiment - the energy value of potatoes
Activity 2 - Investigation of molecule structures using molecular visualisations.
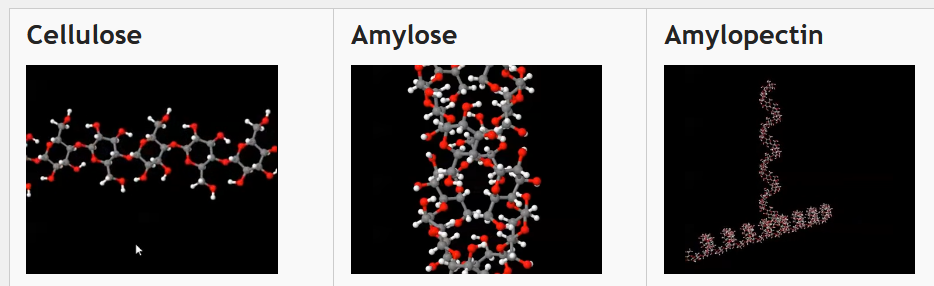
Look at the following carbohydrate macromolecules. Amylose and amylopectin together make starch.
Cellulose
| Amylose
| Amylopectin
| Glycogen
|
Using these online flash cards Quizlet Online activities for this lesson read a little about the functions and structures of these biological macromolecules.
Activity 3 - IB Style questions on carbohydrates
Answer these IB style questions about the biological molecules you have just studied.
- What similarities can you see between the four carbohydrate macromolecules above?
- What is the function of each molecule in living things?
- How does each molecule's structure help the function of the molecule?
Teachers notes
The aim of activity 1 of this lesson is to allow students to complete this experiment which illustrates some of the properties of carbohydrates and lipids, notably energy storage, in the lab. It is an excellent illustration of uncertainties, a useful skill for the IA.
The experiment as described will show that potato cooked in fat (ie a potato crisp) will contain more energy than dried potato (rich in carbohydrates) alone.
Alternatives to the experiment can include using thin card in place of the dried potato. I tried this today and actually got quite reasonable results, see the Teacher only box below. An other possibility could be to use reduced fat crisps.
The experiment would even work if students carefully burn sugar and oils in a spoon under the beaer. The mass of each sample should be measured so that a meaningful comparison can be made.
This is a sample of results of the calorimetry experiment using thin paper card (as a dried potato) and salted crisps.
I used this to model estimations of uncertainty for each variable.
Note: Bio students are not required to propagate the uncertainty into calculated values.
However, I would expect that students realise temperature change, which involves using a thermometer twice will have twice the uncertainty of each individual measurement.
Note also that the table values must have the same number of decimal places as the uncertainty.
There are three small errors of d.p. in the calculated values, and there is also an error in the labels.
Can you spot them? It is often necessary to explain to students that 1g of water has a volume of 1ml.
Click the table to enlarge it.
Activity 2 provides resources which allow students to study the factual theory work about the different molecules as homework or at their own pace one the experiment is complete.
Activity 3 - The IB-style questions can be used to check that the students have understood the simple facts and molecular names. The last question returns to the guiding questions about the carbohydrate molecules.
The link to JSmol 3D visualisations of molecules is a wonderful extension. Without any plugins students can manipulate 3D images of the four macromolecules and see the structures clearly. The short video clips are a simple alternative.

 IB Docs (2) Team
IB Docs (2) Team

