Yeast and Catalase Experiment

Catalase Enzyme and Hydrogen Peroxide.
Following a teacher demonstration of measuring the rate of hydrogen peroxide and catalase enzyme students carry out a circus of three simple enzyme experiments.
These experiments illustrate the effects of Temp, pH and concentration on the enzymes and identify the essential points for the design of experiments in the near future.
Lesson Description
Guiding Question
How can the conditions of an enzyme be changed accurately, and how can the rate of reaction be measured?
Activity 1 Demonstration of Catalase and Hydrogen Peroxide Experiment
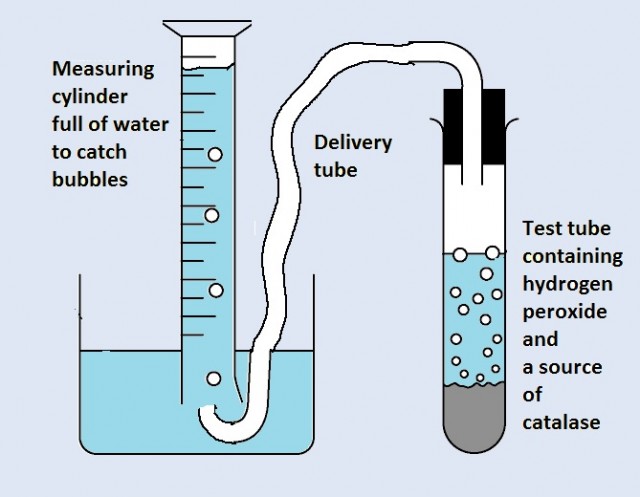
Activity 2 Circus of simple enzyme experiments
Once the method and the set-up is clear, complete the four experiments and answer the questions on the student worksheet below. ![]() Simple enzyme experiments
Simple enzyme experiments
Teachers notes
The experiment is simple, and the students will encounter a few problems while doing each experiment.This will give the students chance to suggest limitations and improvments. It is an opportunity to introduce some skills needed for an IA design on enzymes at a later date.
Review of the experiments, an introduction to designing experimnts for the IA.
This is an activity in planning to help students learn about the IB Design criteria.
Note: This experiment is not suitable for IA assessment, but it is great preparation for IAs in the future.
Investigations of the differences between the different sources of catalase give a good range of possible student investigation. For example, is the optimum pH of catalase the same in yeast and potato? Is the optimum temperature of catalase extracted from liver the same as that extracted from potatoes?
There are a huge range of possibilities for this experiment. The example above does work.
Below are links to other methods using different equipment.

 IB Docs (2) Team
IB Docs (2) Team
